Abstract
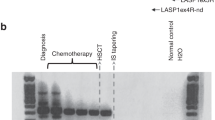
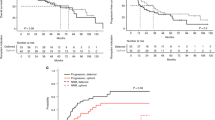
We assessed mammaglobin (MMG) gene expression in bone marrow (BM) aspirates from patients with advanced breast cancer who had received a reduced-intensity conditioning and stem cell allografting, in order to detect a graft-versus-tumor effect on micrometastatic disease. Nine patients received a reduced-intensity conditioning with fludarabine, cyclophosphamide, and thiotepa, followed by peripheral blood allografting from HLA-identical sibling donors. Nested RT-PCR analysis with sequence-specific primers for MMG was carried out on a monthly basis on BM samples. Three patients had MMG-positive BM, four patients had MMG-negative BM before allografting, and two were undetermined. In two patients, a clinical response after allografting (partial remission) occurred concurrently with the clearance of MMG expression, at a median of 6 months after allografting, following immune manipulation. In two patients, a prolonged stable disease and negative MMG expression occurred after day +360 from allografting. In two patients, progression of the disease was associated with MMG RT-PCR changing from negative to positive. In one case, a disease response occurring after donor lymphocyte infusion and grade II acute GVHD was heralded by negativization of MMG expression. Although preliminary, these data suggest that a graft-versus-breast cancer effect is detectable on micrometastatic BM disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Childs R, Chernoff A, Contentin N, Bahceci E, Schrump D, Leitman S et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med 2000; 343: 750–758.
Bregni M, Dodero A, Peccatori J, Pescarollo A, Bernardi M, Sassi I et al. Nonmyeloablative conditioning followed by hematopoietic cell allografting and donor lymphocyte infusions for patients with metastatic renal and breast cancer. Blood 2002; 99: 4234–4236.
Eibl B, Schwaighofer H, Nachbaur D, Marth C, Gachter A, Knapp R et al. Evidence for a graft-versus-tumor effect in a patient treated with marrow ablative chemotherapy and allogeneic bone marrow transplantation for breast cancer. Blood 1996; 88: 1501–1508.
Ueno NT, Rondon G, Mirza NQ, Geisler DK, Anderlini P, Giralt SA et al. Allogeneic peripheral-blood progenitor-cell transplantation for poor-risk patients with metastatic breast cancer. J Clin Oncol 1998; 16: 986–993.
Ueno NT, Cheng YC, Rondon G, Tannir NM, Gajewski JL, Couriel DR et al. Rapid induction of complete donor chimerism by the use of a reduced-intensity conditioning regimen composed of fludarabine and melphalan in allogeneic stem cell transplantation for metastatic solid tumors. Blood 2003; 102: 3829–3836.
Bishop MR, Fowler DH, Marchigiani D, Castro K, Kasten-Sportes C, Steinberg SM et al. Allogeneic lymphocytes induce tumor regression of advanced metastatic breast cancer. J Clin Oncol 2004; 22: 3886–3892.
Bregni M, Bernardi M, Ciceri F, Peccatori J . Allogeneic stem cell transplantation for the treatment of advanced solid tumors. Springer Semin Immunopathol 2004; 26: 95–108.
Dreno B, Nguyen JM, Khammari A, Pandolfino MC, Tessier MH, Bercegeay S et al. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol Immunother 2002; 51: 539–546.
Smith BM, Slade MJ, English J, Graham H, Luchtenborg M, Sinnett HD et al. Response of circulating tumor cells to systemic therapy in patients with metastatic breast cancer: comparison of quantitative polymerase chain reaction and immunocytochemical techniques. J Clin Oncol 2000; 18: 1432–1439.
Diel IJ, Kaufmann M, Costa SD, Holle R, von Minckwitz G, Solomayer EF et al. Micrometastatic breast cancer cells in bone marrow at primary surgery: prognostic value in comparison with nodal status. J Natl Cancer Inst 1996; 88: 1652–1658.
Landys K, Persson S, Kovarik J, Hultborn R, Holmberg E . Prognostic value of bone marrow biopsy in operable breast cancer patients at the time of initial diagnosis: results of a 20-year median follow-up. Breast Cancer Res Treat 1998; 49: 27–33.
Mansi JL, Gogas H, Bliss JM, Gazet JC, Berger U, Coombes RC . Outcome of primary-breast-cancer patients with micrometastases: a long-term follow-up study. Lancet 1999; 354: 197–202.
Vannucchi AM, Bosi A, Glinz S, Pacini P, Linari S, Saccardi R et al. Evaluation of breast tumour cell contamination in the bone marrow and leukapheresis collections by RT-PCR for cytokeratin-19 mRNA. Br J Haematol 1998; 103: 610–617.
Braun S, Cevatli BS, Assemi C, Janni W, Kentenich CR, Schindlbeck C et al. Comparative analysis of micrometastasis to the bone marrow and lymph nodes of node-negative breast cancer patients receiving no adjuvant therapy. J Clin Oncol 2001; 19: 1468–1475.
Gebauer G, Fehm T, Merkle E, Beck EP, Lang N, Jager W . Epithelial cells in bone marrow of breast cancer patients at time of primary surgery: clinical outcome during long-term follow-up. J Clin Oncol 2001; 19: 3669–3674.
Gerber B, Krause A, Muller H, Richter D, Reimer T, Makovitzky J et al. Simultaneous immunohistochemical detection of tumor cells in lymph nodes and bone marrow aspirates in breast cancer and its correlation with other prognostic factors. J Clin Oncol 2001; 19: 960–971.
Braun S, Pantel K, Muller P, Janni W, Hepp F, Kentenich RM et al. Cytokeratin-positive cells in the bone marrow and survival of patients with stage I, II, or III breast cancer. N Engl J Med 2000; 342: 525–533.
Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 2005; 353: 793–802.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004; 351: 781–791.
Pantel K, Schlimok G, Braun S, Kutter D, Lindemann F, Schaller G et al. Differential expression of proliferation-associated molecules in individual micrometastatic carcinoma cells. J Natl Cancer Inst 1993; 85: 1419–1424.
Braun S, Kentenich C, Janni W, Hepp F, de Waal J, Willgeroth F et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol 2000; 18: 80–86.
Braun S, Hepp F, Kentenich CR, Janni W, Pantel K, Riethmuller G et al. Monoclonal antibody therapy with edrecolomab in breast cancer patients: monitoring of elimination of disseminated cytokeratin-positive tumor cells in bone marrow. Clin Cancer Res 1999; 5: 3999–4004.
Braun S, Vogl FD, Janni W, Marth C, Schlimok G, Pantel K . Evaluation of bone marrow in breast cancer patients: prediction of clinical outcome and response to therapy. Breast 2003; 12: 397–404.
Borgen E, Beiske K, Trachsel S, Nesland JM, Kvalheim G, Herstad TK et al. Immunocytochemical detection of isolated epithelial cells in bone marrow: non-specific staining and contribution by plasma cells directly reactive to alkaline phosphatase. J Pathol 1998; 185: 427–434.
Corradini P, Voena C, Astolfi M, Delloro S, Pilotti S, Arrigoni G et al. Maspin and mammaglobin genes are specific markers for RT-PCR detection of minimal residual disease in patients with breast cancer. Ann Oncol 2001; 12: 1693–1698.
Slade MJ, Smith BM, Sinnett HD, Cross NC, Coombes RC . Quantitative polymerase chain reaction for the detection of micrometastases in patients with breast cancer. J Clin Oncol 1999; 17: 870–879.
Suchy B, Austrup F, Driesel G, Eder C, Kusiak I, Uciechowski P et al. Detection of mammaglobin expressing cells in blood of breast cancer patients. Cancer Lett 2000; 158: 171–178.
Franklin WA, Shpall EJ, Archer P, Johnston CS, Garza-Williams S, Hami L et al. Immunocytochemical detection of breast cancer cells in marrow and peripheral blood of patients undergoing high dose chemotherapy with autologous stem cell support. Breast Cancer Res Treat 1996; 41: 1–13.
Pecora AL, Lazarus HM, Jennis AA, Preti RA, Goldberg SL, Rowley SD et al. Breast cancer cell contamination of blood stem cell products in patients with metastatic breast cancer: predictors and clinical relevance. Biol Blood Marrow Transplant 2002; 8: 536–543.
Ferrucci PF, Rabascio C, Mazzetta C, Cocorocchio E, Agazzi A, Vanazzi A et al. Mammaglobin expression in leukapheresis products is a predictive marker of poor prognosis in women with high-risk breast cancer. Clin Cancer Res 2004; 10: 6039–6046.
Borgen E, Naume B, Nesland JM, Kvalheim G, Beiske K, Fodstad O et al. Standardization of the immunocytochemical detection of cancer cells in bone marrow and blood. I. Establishment of objective criteria for the evaluation of immunostained cells. Cytotherapy 1999; 1: 377–388.
Carella AM, Beltrami G, Corsetti MT, Nati S, Musto P, Scalzulli P et al. Reduced intensity conditioning for allograft after cytoreductive autograft in metastatic breast cancer. Lancet 2005; 366: 318–320.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bregni, M., Fleischhauer, K., Bernardi, M. et al. Bone marrow mammaglobin expression as a marker of graft-versus-tumor effect after reduced-intensity allografting for advanced breast cancer. Bone Marrow Transplant 37, 311–315 (2006). https://doi.org/10.1038/sj.bmt.1705248
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705248