Abstract
Developments in synthetic chemistry are increasingly driven by improvements in the selectivity and sustainability of transformations. Bifunctional reagents, either as dual coupling partners or as a coupling partner in combination with an activating species, offer an atom-economic approach to chemical complexity, while suppressing the formation of waste. These reagents are employed in organic synthesis thanks to their ability to form complex organic architectures and empower novel reaction pathways. This Review describes several key bifunctional reagents by showcasing selected cornerstone research areas and examples, including radical reactions, C–H functionalization, cross-coupling, organocatalysis and cyclization reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
References
Ball, P. Chemistry: why synthesize? Nature 528, 327–329 (2015).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Campos, K. R. et al. The importance of synthetic chemistry in the pharmaceutical industry. Science 363, eaat0805 (2019).
Trost, B. M. The atom economy–a search for synthetic efficiency. Science 254, 1471–1477 (1991). An early perspective that describes the concept of atom-economy in synthetic chemistry.
Wender, P. A. & Miller, B. L. Synthesis at the molecular frontier. Nature 460, 197–201 (2009).
Young, I. S. & Baran, P. S. Protecting-group-free synthesis as an opportunity for invention. Nat. Chem. 1, 193–205 (2009).
Piers, E. The use of some bifunctional reagents in organic synthesis. Pure Appl. Chem. 60, 107–114 (1988). A seminal review regarding the concept of ‘bifunctional reagents’.
Yan, M., Lo, J. C., Edwards, J. T. & Baran, P. S. Radicals: reactive intermediates with translational potential. J. Am. Chem. Soc. 138, 12692–12714 (2016). An excellent perspective that describes the history and development of radical chemistry.
Studer, A. & Curran, D. P. Catalysis of radical reactions: a radical chemistry perspective. Angew. Chem. Int. Ed. 55, 58–102 (2016).
Jasperse, C. P., Curran, D. P. & Fevig, T. L. Radical reactions in natural product synthesis. Chem. Rev. 91, 1237–1286 (1991).
Renaud, P. & Sibi, M. P. Radicals in Organic Synthesis (Wiley, 2001).
Zard, S. Z. Radical Reactions in Organic Synthesis (Oxford Univ. Press, 2003).
Huang, H.-M., Garduño-Castro, M. H., Morrill, C. & Procter, D. J. Catalytic cascade reactions by radical relay. Chem. Soc. Rev. 48, 4626–4638 (2019).
Plesniak, M. P., Huang, H.-M. & Procter, D. J. Radical cascade reactions triggered by single electron transfer. Nat. Rev. Chem. 1, 0077 (2017).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013). An excellent review on photoredox chemistry in synthetic chemistry.
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
McAtee, R. C., McClain, E. J. & Stephenson, C. R. J. Illuminating photoredox catalysis. Trends Chem. 1, 111–125 (2019).
Tellis, J. C. et al. Single-electron transmetalation via photoredox/nickel dual catalysis: unlocking a new paradigm for sp3–sp2 cross-coupling. Acc. Chem. Res. 49, 1429–1439 (2016).
Yu, X.-Y., Zhao, Q.-Q., Chen, J., Xiao, W.-J. & Chen, J.-R. When light meets nitrogen-centered radicals: from reagents to catalysts. Acc. Chem. Res. 53, 1066–1083 (2020).
Yan, M., Kawamata, Y. & Baran, P. S. Synthetic organic electrochemical methods since 2000: on the verge of a renaissance. Chem. Rev. 117, 13230–13319 (2017).
Yuan, Y. & Lei, A. Electrochemical oxidative cross-coupling with hydrogen evolution reactions. Acc. Chem. Res. 52, 3309–3324 (2019).
Xiong, P. & Xu, H.-C. Chemistry with electrochemically generated N-centered radicals. Acc. Chem. Res. 52, 3339–3350 (2019).
Kingston, C. et al. A survival guide for the “electro-curious”. Acc. Chem. Res. 53, 72–83 (2020).
Kharasch, M. S., Jensen, E. V. & Urry, W. H. Addition of carbon tetrachloride and chloroform to olefins. Science 102, 128–128 (1945).
Curran, D. P. The design and application of free radical chain reactions in organic synthesis. Part 2. Synthesis 1988, 489–513 (1988).
Pintauer, T. & Matyjaszewski, K. Atom transfer radical addition and polymerization reactions catalyzed by ppm amounts of copper complexes. Chem. Soc. Rev. 37, 1087–1097 (2008).
Nguyen, J. D., Tucker, J. W., Konieczynska, M. D. & Stephenson, C. R. J. Intermolecular atom transfer radical addition to olefins mediated by oxidative quenching of photoredox catalysts. J. Am. Chem. Soc. 133, 4160–4163 (2011). First example of photocatalytic atom transfer radical addition reactions.
Wallentin, C.-J., Nguyen, J. D., Finkbeiner, P. & Stephenson, C. R. J. Visible light-mediated atom transfer radical addition via oxidative and reductive quenching of photocatalysts. J. Am. Chem. Soc. 134, 8875–8884 (2012).
Pirtsch, M., Paria, S., Matsuno, T., Isobe, H. & Reiser, O. [Cu(dap)2Cl] As an efficient visible-light-driven photoredox catalyst in carbon–carbon bond-forming reactions. Chem. Eur. J. 18, 7336–7340 (2012).
Arceo, E., Montroni, E. & Melchiorre, P. Photo-organocatalysis of atom-transfer radical additions to alkenes. Angew. Chem. Int. Ed. 53, 12064–12068 (2014).
Qin, Q., Ren, D. & Yu, S. Visible-light-promoted chloramination of olefins with N-chlorosulfonamide as both nitrogen and chlorine sources. Org. Biomol. Chem. 13, 10295–10298 (2015).
Magagnano, G. et al. Photocatalytic ATRA reaction promoted by iodo-Bodipy and sodium ascorbate. Chem. Commun. 53, 1591–1594 (2017).
Li, H., Shan, C., Tung, C.-H. & Xu, Z. Dual gold and photoredox catalysis: visible light-mediated intermolecular atom transfer thiosulfonylation of alkenes. Chem. Sci. 8, 2610–2615 (2017).
Zhu, D., Shao, X., Hong, X., Lu, L. & Shen, Q. PhSO2SCF2H: a shelf-stable, easily scalable reagent for radical difluoromethylthiolation. Angew. Chem. Int. Ed. 55, 15807–15811 (2016).
Li, H., Cheng, Z., Tung, C.-H. & Xu, Z. Atom transfer radical addition to alkynes and enynes: a versatile gold/photoredox approach to thio-functionalized vinylsulfones. ACS Catal 8, 8237–8243 (2018).
Jiang, H. & Studer, A. Intermolecular radical carboamination of alkenes. Chem. Soc. Rev. 49, 1790–1811 (2020).
Weidner, K., Giroult, A., Panchaud, P. & Renaud, P. Efficient carboazidation of alkenes using a radical desulfonylative azide transfer process. J. Am. Chem. Soc. 132, 17511–17515 (2010).
Monos, T. M., McAtee, R. C. & Stephenson, C. R. J. Arylsulfonylacetamides as bifunctional reagents for alkene aminoarylation. Science 361, 1369–1373 (2018). This paper describes arylsulfonylacetamides as bifunctional reagents as applied in radical chemistry.
Zhang, Y. et al. Intermolecular carboamination of unactivated alkenes. J. Am. Chem. Soc. 140, 10695–10699 (2018).
Patra, T., Bellotti, P., Strieth-Kalthoff, F. & Glorius, F. Photosensitized intermolecular carboimination of alkenes through the persistent radical effect. Angew. Chem. Int. Ed. 59, 3172–3177 (2020).
Soni, V. K. et al. Reactivity tuning for radical–radical cross-coupling via selective photocatalytic energy transfer: access to amine building blocks. ACS Catal. 9, 10454–10463 (2019).
Okada, K., Okamoto, K., Morita, N., Okubo, K. & Oda, M. Photosensitized decarboxylative Michael addition through N-(acyloxy)phthalimides via an electron-transfer mechanism. J. Am. Chem. Soc. 113, 9401–9402 (1991).
Qin, T. et al. A general alkyl-alkyl cross-coupling enabled by redox-active esters and alkylzinc reagents. Science 352, 801–805 (2016).
Huang, H.-M. et al. Catalytic radical generation of π-allylpalladium complexes. Nat. Catal. 3, 393–400 (2020).
Huang, H.-M. et al. Three-component, interrupted radical Heck/allylic substitution cascade involving unactivated alkyl bromides. J. Am. Chem. Soc. 142, 10173–10183 (2020).
Phelan, J. P. et al. Redox-neutral photocatalytic cyclopropanation via radical/polar crossover. J. Am. Chem. Soc. 140, 8037–8047 (2018).
Kim, Y., Lee, K., Mathi, G. R., Kim, I. & Hong, S. Visible-light-induced cascade radical ring-closure and pyridylation for the synthesis of tetrahydrofurans. Green. Chem. 21, 2082–2087 (2019).
Moon, Y. et al. Visible light induced alkene aminopyridylation using N-aminopyridinium salts as bifunctional reagents. Nat. Commun. 10, 4117 (2019). Excellent example of radical chemistry involving N-aminopyridinium salts as bifunctional reagents.
Mathi, G. R., Jeong, Y., Moon, Y. & Hong, S. Photochemical carbopyridylation of alkenes using N-alkenoxypyridinium salts as bifunctional reagents. Angew. Chem. Int. Ed. 59, 2049–2054 (2020).
Kim, I. et al. Visible-light-induced pyridylation of remote C(sp3)−H bonds by radical translocation of N-alkoxypyridinium salts. Angew. Chem. Int. Ed. 57, 15517–15522 (2018).
Kim, N., Lee, C., Kim, T. & Hong, S. Visible-light-induced remote C(sp3)–H pyridylation of sulfonamides and carboxamides. Org. Lett. 21, 9719–9723 (2019).
Kim, I. et al. Site-selective functionalization of pyridinium derivatives via visible-light-driven photocatalysis with quinolinone. J. Am. Chem. Soc. 141, 9239–9248 (2019).
Jung, S., Lee, H., Moon, Y., Jung, H.-Y. & Hong, S. Site-selective C–H acylation of pyridinium derivatives by photoredox catalysis. ACS Catal. 9, 9891–9896 (2019).
Lee, K., Lee, S., Kim, N., Kim, S. & Hong, S. Visible-light-enabled trifluoromethylative pyridylation of alkenes from pyridines and triflic anhydride. Angew. Chem. Int. Ed. 59, 13379–13384 (2020).
Moon, Y., Lee, W. & Hong, S. Visible-light-enabled ortho-Selective aminopyridylation of alkenes with N-aminopyridinium ylides. J. Am. Chem. Soc. 142, 12420–12429 (2020).
Quiclet-Sire, B. & Zard, S. Z. Fun with radicals: Some new perspectives for organic synthesis. Pure Appl. Chem. 83, 519–551 (2010).
Zard, S. Z. The xanthate route to ketones: when the radical is better than the enolate. Acc. Chem. Res. 51, 1722–1733 (2018).
Lopez-Ruiz, H. & Zard, S. Z. A flexible access to highly functionalised boronates. Chem. Commun. 2618–2619 (2001).
Quiclet-Sire, B. & Zard, S. Z. Radical instability in aid of efficiency: a powerful route to highly functional MIDA boronates. J. Am. Chem. Soc. 137, 6762–6765 (2015).
Li, S.-G., Portela-Cubillo, F. & Zard, S. Z. A convergent synthesis of enantiopure open-chain, cyclic, and fluorinated α-amino acids. Org. Lett. 18, 1888–1891 (2016).
Legrand, N., Quiclet-Sire, B. & Zard, S. Z. Radical addition to strained olefins: a flexible access to small ring derivatives. Tetrahedron Lett 41, 9815–9818 (2000).
Bacqué, E., Pautrat, F. & Zard, S. Z. A flexible strategy for the divergent modification of pleuromutilin. Chem. Commun. 2312–2313 (2002).
Bagal, D. B. et al. Trifluoromethylchlorosulfonylation of alkenes: evidence for an inner-sphere mechanism by a copper phenanthroline photoredox catalyst. Angew. Chem. Int. Ed. 54, 6999–7002 (2015).
Tanaka, S., Nakayama, Y., Konishi, Y., Koike, T. & Akita, M. Fluoroalkanesulfinate salts as dual fluoroalkyl and SO2 sources: atom-economical fluoroalkyl-sulfonylation of alkenes and alkynes by photoredox catalysis. Org. Lett. 22, 2801–2805 (2020).
Li, Z. et al. CF3SO2Na as a bifunctional reagent: electrochemical trifluoromethylation of alkenes accompanied by SO2 insertion to access trifluoromethylated cyclic N-sulfonylimines. Angew. Chem. Int. Ed. 59, 7266–7270 (2020).
Kondo, M. et al. Silaboration of [1.1.1]propellane: a storable feedstock for bicyclo[1.1.1]pentane derivatives. Angew. Chem. Int. Ed. 59, 1970–1974 (2020).
Wu, Z., Xu, Y., Liu, J., Wu, X. & Zhu, C. A practical access to fluoroalkylthio(seleno)-functionalized bicyclo[1.1.1]pentanes. Sci. China Chem. 63, 1025–1029 (2020).
Gillis, E. P., Eastman, K. J., Hill, M. D., Donnelly, D. J. & Meanwell, N. A. Applications of fluorine in medicinal chemistry. J. Med. Chem. 58, 8315–8359 (2015).
Muller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Qiu, S., Xu, T., Zhou, J., Guo, Y. & Liu, G. Palladium-catalyzed intermolecular aminofluorination of styrenes. J. Am. Chem. Soc. 132, 2856–2857 (2010).
Zhang, H., Song, Y., Zhao, J., Zhang, J. & Zhang, Q. Regioselective radical aminofluorination of styrenes. Angew. Chem. Int. Ed. 53, 11079–11083 (2014).
Abrams, D. J., Provencher, P. A. & Sorensen, E. J. Recent applications of C–H functionalization in complex natural product synthesis. Chem. Soc. Rev. 47, 8925–8967 (2018).
Liu, C. et al. Oxidative coupling between two hydrocarbons: an update of recent C–H functionalizations. Chem. Rev. 115, 12138–12204 (2015).
Gensch, T., Hopkinson, M. N., Glorius, F. & Wencel-Delord, J. Mild metal-catalyzed C–H activation: examples and concepts. Chem. Soc. Rev. 45, 2900–2936 (2016).
Modha, S. G. & Greaney, M. F. Atom-economical transformation of diaryliodonium salts: tandem C–H and N–H arylation of indoles. J. Am. Chem. Soc. 137, 1416–1419 (2015). A seminal work on atom-economic transformation of diaryliodonium salts.
Teskey, C. J., Sohel, S. M. A., Bunting, D. L., Modha, S. G. & Greaney, M. F. Domino N-/C-arylation via in situ generation of a directing group: atom-efficient arylation using diaryliodonium salts. Angew. Chem. Int. Ed. 56, 5263–5266 (2017).
Li, S. et al. Domino N-/C-or N-/N-/C-arylation of imidazoles to yield polyaryl imidazolium salts via atom-economical use of diaryliodonium salts. Chem. Commun. 55, 11267–11270 (2019).
Lerchen, A., Knecht, T., Daniliuc, C. G. & Glorius, F. Unnatural amino acid synthesis enabled by the regioselective cobalt(III)-catalyzed intermolecular carboamination of alkenes. Angew. Chem. Int. Ed. 55, 15166–15170 (2016).
Johansson Seechurn, C. C. C., Kitching, M. O., Colacot, T. J. & Snieckus, V. Palladium-catalyzed cross-coupling: a historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 51, 5062–5085 (2012).
Biffis, A., Centomo, P., Del Zotto, A. & Zecca, M. Pd Metal catalysts for cross-couplings and related reactions in the 21st century: a critical review. Chem. Rev. 118, 2249–2295 (2018).
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C–N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
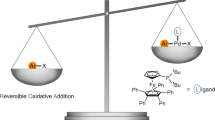
Jones, D. J., Lautens, M. & McGlacken, G. P. The emergence of Pd-mediated reversible oxidative addition in cross coupling, carbohalogenation and carbonylation reactions. Nat. Catal. 2, 843–851 (2019).
Zhdankin, V. V. & Stang, P. J. Chemistry of polyvalent iodine. Chem. Rev. 108, 5299–5358 (2008).
Chatterjee, N. & Goswami, A. Synthesis and application of cyclic diaryliodonium salts: a platform for bifunctionalization in a single step. Eur. J. Org. Chem. 3023–3032 (2017).
Chen, H., Han, J. & Wang, L. Intramolecular aryl migration of diaryliodonium salts: access to ortho-iodo diaryl ethers. Angew. Chem. Int. Ed. 57, 12313–12317 (2018).
Liang, Y., Jing, H., Liu, C., Wu, X. & Ma, Y. Stereoselective palladium-catalyzed coupling of 3,7-bis(N,N-dimethylamino)-10H-dibenz[b,e]iodinium iodide with α,β-unsaturated carbonyl compounds. Tetrahedron Lett. 39, 7143–7146 (1998).
Kina, A., Miki, H., Cho, Y.-H. & Hayashi, T. Palladium-catalyzed Heck and carbonylation reactions of a dinaphthaleneiodonium salt forming functionalized 2-iodo-1,1′-binaphthyls. Adv. Synth. Catal. 346, 1728–1732 (2004).
Xu, S., Zhao, K. & Gu, Z. Copper-catalyzed asymmetric ring-opening of cyclic diaryliodonium with benzylic and aliphatic amines. Adv. Synth. Catal. 360, 3877–3883 (2018).
Zhao, K. et al. Enhanced reactivity by torsional strain of cyclic diaryliodonium in Cu-catalyzed enantioselective ring-opening reaction. Chem 4, 599–612 (2018).
Hou, M., Deng, R. & Gu, Z. Cu-catalyzed enantioselective atropisomer synthesis via thiolative ring opening of five-membered cyclic diaryliodoniums. Org. Lett. 20, 5779–5783 (2018).
Li, Q., Zhang, M., Zhan, S. & Gu, Z. Copper-catalyzed enantioselective ring-opening of cyclic diaryliodoniums and O-alkylhydroxylamines. Org. Lett. 21, 6374–6377 (2019).
Duan, L., Zhao, K., Wang, Z., Zhang, F.-L. & Gu, Z. Enantioselective ring-opening/oxidative phosphorylation and P-transfer reaction of cyclic diaryliodoniums. ACS Catal. 9, 9852–9858 (2019).
Zhu, K. et al. Enantioselective synthesis of axially chiral biaryls via Cu-catalyzed acyloxylation of cyclic diaryliodonium salts. ACS Catal. 9, 4951–4957 (2019).
Zhu, K., Wang, Y., Fang, Q., Song, Z. & Zhang, F. Enantioselective synthesis of axially chiral biaryls via copper-catalyzed thiolation of cyclic diaryliodonium salts. Org. Lett. 22, 1709–1713 (2020).
Li, B., Chao, Z., Li, C. & Gu, Z. Cu-catalyzed enantioselective ring opening of cyclic diaryliodoniums toward the synthesis of chiral diarylmethanes. J. Am. Chem. Soc. 140, 9400–9403 (2018).
Miralles, N., Romero, R. M., Fernández, E. & Muñiz, K. A mild carbon–boron bond formation from diaryliodonium salts. Chem. Commun. 51, 14068–14071 (2015).
Suero, M. G., Bayle, E. D., Collins, B. S. L. & Gaunt, M. J. Copper-catalyzed electrophilic carbofunctionalization of alkynes to highly functionalized tetrasubstituted alkenes. J. Am. Chem. Soc. 135, 5332–5335 (2013). This paper describes copper-catalysed electrophilic carbofunctionalization of alkynes with vinyliodonium and diaryliodonium triflates as bifunctional reagents.
Wang, X. & Studer, A. Regio-and stereoselective cyanotriflation of alkynes using aryl(cyano)iodonium triflates. J. Am. Chem. Soc. 138, 2977–2980 (2016).
Wang, X. & Studer, A. Regio-and stereoselective radical perfluoroalkyltriflation of alkynes using phenyl(perfluoroalkyl)iodonium triflates. Org. Lett. 19, 2977–2980 (2017).
Hari, D. P. & Waser, J. Enantioselective copper-catalyzed oxy-alkynylation of diazo compounds. J. Am. Chem. Soc. 139, 8420–8423 (2017). This paper describes highly enantioselective oxyalkynylation of diazo compounds using ethynylbenziodoxol-(on)e reagents as bifunctional reagents.
Borrel, J., Pisella, G. & Waser, J. Copper-catalyzed oxyalkynylation of C–S bonds in thiiranes and thiethanes with hypervalent iodine reagents. Org. Lett. 22, 422–427 (2020).
Modha, S. G., Popescu, M. V. & Greaney, M. F. Synthesis of triarylamines via sequential C–N bond formation. J. Org. Chem. 82, 11933–11938 (2017).
Hu, J., Xie, Y. & Huang, H. Palladium-catalyzed insertion of an allene into an aminal: aminomethylamination of allenes by C–N bond activation. Angew. Chem. Int. Ed. 53, 7272–7276 (2014).
Qin, G., Li, L., Li, J. & Huang, H. Palladium-catalyzed formal insertion of carbenoids into aminals via C–N bond activation. J. Am. Chem. Soc. 137, 12490–12493 (2015).
Wang, W. & Huang, H. Palladium-catalyzed formal insertion of carbenoids into N,O-aminals: direct access to α-alkoxy-β-amino acid esters. Chem. Commun. 55, 3947–3950 (2019).
Yu, J., Chen, L. & Sun, J. Copper-catalyzed oxy-aminomethylation of diazo compounds with N,O-acetals. Org. Lett. 21, 1664–1667 (2019).
Yu, J., Xu, G., Tang, S., Shao, Y. & Sun, J. Copper-catalyzed amino-oxymethylation of ynamides with N,O-acetals. Org. Lett. 21, 9076–9079 (2019).
Nozaki, K., Sato, N. & Takaya, H. Acylcyanation of terminal acetylenes: palladium-catalyzed addition of aryloyl cyanides to arylacetylenes. J. Org. Chem. 59, 2679–2681 (1994).
Suginome, M., Yamamoto, A. & Murakami, M. Palladium-and nickel-catalyzed intramolecular cyanoboration of alkynes. J. Am. Chem. Soc. 125, 6358–6359 (2003).
Miyazaki, Y., Ohta, N., Semba, K. & Nakao, Y. Intramolecular aminocyanation of alkenes by cooperative palladium/boron catalysis. J. Am. Chem. Soc. 136, 3732–3735 (2014).
Pan, Z., Wang, S., Brethorst, J. T. & Douglas, C. J. Palladium and Lewis-acid-catalyzed intramolecular aminocyanation of alkenes: scope, mechanism, and stereoselective alkene difunctionalizations. J. Am. Chem. Soc. 140, 3331–3338 (2018).
Koester, D. C., Kobayashi, M., Werz, D. B. & Nakao, Y. Intramolecular oxycyanation of alkenes by cooperative Pd/BPh3 catalysis. J. Am. Chem. Soc. 134, 6544–6547 (2012).
Watson, M. P. & Jacobsen, E. N. Asymmetric intramolecular arylcyanation of unactivated olefins via C−CN bond activation. J. Am. Chem. Soc. 130, 12594–12595 (2008).
Zhang, T., Luan, Y.-X. Y., Zheng, S.-J., Peng, Q. & Ye, M. Chiral aluminum complex controls enantioselective nickel-catalyzed synthesis of indenes: C−CN bond activation. Angew. Chem. Int. Ed. 59, 7439–7443 (2020).
Chatani, N. & Hanafusa, T. Palladium-catalysed addition of trimethylsilyl cyanide to arylacetylenes. J. Chem. Soc. Chem. Commun. 838–839 (1985).
Chatani, N., Horiuchi, N. & Hanafusa, T. Palladium-catalyzed addition of trimethylgermyl cyanide to terminal acetylenes. J. Org. Chem. 55, 3393–3395 (1990).
Nakao, Y., Hirata, Y. & Hiyama, T. Cyanoesterification of 1,2-dienes: synthesis and transformations of highly functionalized α-cyanomethylacrylate esters. J. Am. Chem. Soc. 128, 7420–7421 (2006).
Hirata, Y. et al. Nickel/Lewis acid-catalyzed cyanoesterification and cyanocarbamoylation of alkynes. J. Am. Chem. Soc. 132, 10070–10077 (2010).
Nakao, Y., Yada, A., Ebata, S. & Hiyama, T. A dramatic effect of Lewis-acid catalysts on nickel-catalyzed carbocyanation of alkynes. J. Am. Chem. Soc. 129, 2428–2429 (2007).
Nakao, Y., Yada, A. & Hiyama, T. Heteroatom-directed alkylcyanation of alkynes. J. Am. Chem. Soc. 132, 10024–10026 (2010).
Hirata, Y., Yukawa, T., Kashihara, N., Nakao, Y. & Hiyama, T. Nickel-catalyzed carbocyanation of alkynes with allyl cyanides. J. Am. Chem. Soc. 131, 10964–10973 (2009).
Piou, T. & Rovis, T. Rhodium-catalysed syn-carboamination of alkenes via a transient directing group. Nature 527, 86–90 (2015). This paper describes rhodium-catalysed carboamination of alkenes at the same (syn) face of a double bond, initiated by a carbon–hydrogen activation event that uses enoxyphthalimides as the source of both the carbon and the nitrogen functionalities.
Rabet, P. T. G., Boyd, S. & Greaney, M. F. Metal-free intermolecular aminoarylation of alkyne. Angew. Chem. Int. Ed. 56, 4183–4186 (2017).
Han, D., He, Q. & Fan, R. Formal group insertion into aryl C–N bonds through an aromaticity destruction-reconstruction process. Nat. Commun. 9, 3423 (2018).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
He, M. & Bode, J. W. Enantioselective, NHC-catalyzed bicyclo-β-lactam formation via direct annulations of enals and unsaturated N-sulfonyl ketimines. J. Am. Chem. Soc. 130, 418–419 (2008).
Candish, L. & Lupton, D. W. N-heterocyclic carbene-catalyzed Ireland–Coates Claisen rearrangement: synthesis of functionalized β-lactones. J. Am. Chem. Soc. 135, 58–61 (2013).
Raup, D. E. A., Cardinal-David, B., Holte, D. & Scheidt, K. A. Cooperative catalysis by carbenes and Lewis acids in a highly stereoselective route to γ-lactams. Nat. Chem. 2, 766–771 (2010). This paper describes a cooperative NHC/Lewis acid catalytic system promoting the addition of homoenolate equivalents to hydrazones, generating highly substituted γ-lactams.
Zhao, X., DiRocco, D. A. & Rovis, T. N-heterocyclic carbene and Brønsted acid cooperative catalysis: asymmetric synthesis of trans-γ-lactams. J. Am. Chem. Soc. 133, 12466–12469 (2011).
Lee, A. et al. Enantioselective annulations for dihydroquinolones by in situ generation of azolium enolates. J. Am. Chem. Soc. 136, 10589–10592 (2014).
Lv, H., Jia, W.-Q., Sun, L.-H. & Ye, S. N-heterocyclic carbene catalyzed [4+3] annulation of enals and o-quinone methides: highly enantioselective synthesis of benzo-ε-lactones. Angew. Chem. Int. Ed. 52, 8607–8610 (2013).
Izquierdo, J., Orue, A. & Scheidt, K. A. A dual Lewis base activation strategy for enantioselective carbene-catalyzed annulations. J. Am. Chem. Soc. 135, 10634–10637 (2013).
Wang, L. et al. Asymmetric synthesis of spirobenzazepinones with atroposelectivity and spiro-1,2-diazepinones by NHC-catalyzed [3+4] annulation reactions. Angew. Chem. Int. Ed. 55, 11110–11114 (2016).
Guo, C., Fleige, M., Janssen-Müller, D., Daniliuc, C. G. & Glorius, F. Cooperative N-heterocyclic carbene/palladium-catalyzed enantioselective umpolung annulations. J. Am. Chem. Soc. 138, 7840–7843 (2016).
Singha, S., Patra, T., Daniliuc, C. G. & Glorius, F. Highly enantioselective [5 + 2] annulations through cooperative N-heterocyclic carbene (NHC) organocatalysis and palladium catalysis. J. Am. Chem. Soc. 140, 3551–3554 (2018).
Singha, S., Serrano, E., Mondal, S., Daniliuc, C. G. & Glorius, F. Diastereodivergent synthesis of enantioenriched α,β-disubstituted γ-butyrolactones via cooperative N-heterocyclic carbene and Ir catalysis. Nat. Catal. 3, 48–54 (2020).
Ma, S. Handbook of Cyclization Reactions (Wiley, 2003).
Barluenga, J., Sanz, R., Granados, A. & Fañanás, F. J. First intramolecular carbometalation of lithiated double bonds. A new synthesis of functionalized indoles and dihydropyrroles. J. Am. Chem. Soc. 120, 4865–4866 (1998).
Xi, Z. 1,4-Dilithio-1,3-dienes: reaction and synthetic applications. Acc. Chem. Res. 43, 1342–1351 (2010).
Yu, N. et al. Diverse reactions of 1,4-dilithio-1,3-dienes with nitriles: facile access to tricyclic Δ1-bipyrrolines, multiply substituted pyridines, siloles, and (Z,Z)-dienylsilanes by tuning of substituents on the butadienyl skeleton. Chem. Eur. J. 14, 5670–5679 (2008).
Fang, H., Li, G., Mao, G. & Xi, Z. Reactions of substituted (1,3-butadiene-1,4-diyl)magnesium, 1,4-bis(bromomagnesio)butadienes and 1,4-dilithiobutadienes with ketones, aldehydes and PhNO to yield cyclopentadiene derivatives and N-Ph pyrroles by cyclodialkenylation. Chem. Eur. J. 10, 3444–3450 (2004).
Wei, J. et al. Magnesiacyclopentadienes as alkaline-earth metallacyclopentadienes: facile synthesis, structural characterization, and synthetic application. Angew. Chem. Int. Ed. 53, 5634–5638 (2014).
Xi, Z., Song, Q., Chen, J., Guan, H. & Li, P. Dialkenylation of carbonyl groups by alkenyllithium compounds: formation of cyclopentadiene derivatives by the reaction of 1,4-dilithio-1,3-dienes with ketones and aldehydes. Angew. Chem. Int. Ed. 40, 1913–1916 (2001).
Wang, C. et al. Metal-mediated efficient synthesis, structural characterization, and skeletal rearrangement of octasubstituted semibullvalenes. J. Am. Chem. Soc. 128, 4564–4565 (2006).
Chen, J. et al. Stereoselective synthesis of polysubstituted 2,5-dihydrofurans from reaction of 1,4-dilithio-1,3-dienes with aldehydes. Org. Lett. 4, 2269–2271 (2002).
Fischer, C. & Sparr, C. Direct transformation of esters into heterocyclic fluorophores. Angew. Chem. Int. Ed. 57, 2436–2440 (2018).
Link, A., Fischer, C. & Sparr, C. Direct transformation of esters into arenes with 1,5-bifunctional organomagnesium reagents. Angew. Chem. Int. Ed. 54, 12163–12166 (2015).
Link, A. & Sparr, C. Remote central-to-axial chirality conversion: direct atroposelective ester to biaryl transformation. Angew. Chem. Int. Ed. 57, 7136–7139 (2018). A strategy for remote central-to-axial chirality conversion by simultaneous planarization of an encoding and transient stereocentre is presented in this paper.
Zhu, C., Xu, G. & Sun, J. Gold-catalyzed formal [4+1]/[4+3] cycloadditions of diazo esters with triazines. Angew. Chem. Int. Ed. 55, 11867–11871 (2016).
Zeng, Z. et al. Gold-catalyzed intermolecular cyclocarboamination of ynamides with 1,3,5-triazinanes: en route to tetrahydropyrimidines. Chem. Commun. 53, 4304–4307 (2017).
Garve, L. K. B., Jones, P. G. & Werz, D. B. Ring-opening 1-amino-3-aminomethylation of donor–acceptor cyclopropanes via 1,3-diazepanes. Angew. Chem. Int. Ed. 56, 9226–9230 (2017).
Peng, S., Ji, D. & Sun, J. Gold-catalyzed [2+2+2+2]-annulation of 1,3,5-hexahydro-1,3,5-triazines with alkoxyallenes. Chem. Commun. 53, 12770–12773 (2017).
Ge, J., Wu, X. & Bao, X. Rhodium(II)-catalyzed annulation of N-sulfonyl-1,2,3-triazoles with 1,3,5-triazinanes to produce octahydro-1 H-purine derivatives: a combined experimental and computational study. Chem. Commun. 55, 6090–6093 (2019).
Shimizu, M. et al. Copper-catalyzed double S-arylation of potassium thioacetate with dibenziodolium triflates: facile synthesis of unsymmetrical dibenzothiophenes. Eur. J. Org. Chem. 2785–2788 (2016).
Zhu, D. et al. Synthesis of carbazoles via one-pot copper-catalyzed amine insertion into cyclic diphenyleneiodoniums as a strategy to generate a drug-like chemical library. Adv. Synth. Catal. 355, 2172–2178 (2013).
Riedmüller, S. & Nachtsheim, B. J. Palladium-catalyzed synthesis of N-arylated carbazoles using anilines and cyclic diaryliodonium salts. Beilstein J. Org. Chem. 9, 1202–1209 (2013).
Wu, Y. et al. Pd catalyzed insertion of alkynes into cyclic diaryliodoniums: a direct access to multi-substituted phenanthrenes. Org. Biomol. Chem. 13, 10386–10391 (2015).
Wu, Y. et al. Palladium catalyzed dual C–H functionalization of indoles with cyclic diaryliodoniums, an approach to ring fused carbazole derivatives. Org. Biomol. Chem. 12, 9777–9780 (2014).
Ye, Z., Li, Y., Xu, K., Chen, N. & Zhang, F. Cascade π-extended decarboxylative annulation involving cyclic diaryliodonium salts: site-selective synthesis of phenanthridines and benzocarbazoles via a traceless directing group strategy. Org. Lett. 21, 9869–9873 (2019).
Hu, T. et al. Synthesis of tribenzo[b,d,f]azepines via cascade π-extended decarboxylative annulation involving cyclic diaryliodonium salts. Org. Lett. 22, 505–509 (2020).
Liu, Z. et al. Mild Cu(I)-catalyzed cascade reaction of cyclic diaryliodoniums, sodium azide, and alkynes: efficient synthesis of triazolophenanthridines. Org. Lett. 16, 5600–5603 (2014).
Zhu, D. et al. Three-component Pd/Cu-catalyzed cascade reactions of cyclic iodoniums, alkynes, and boronic acids: an approach to methylidenefluorenes. Org. Lett. 16, 2350–2353 (2014).
Liu, Z. et al. Cu/Pd-catalyzed cascade reactions of cyclic diaryliodoniums and alkynes – access to fluorenes with conjugate enynes/dienes. Eur. J. Org. Chem. 1110–1118 (2016).
Zhu, D. et al. Relayed regioselective alkynylation/olefination of unsymmetrical cyclic diaryliodonium species catalyzed by Cu and Pd: affording fluorescent cytotoxic benzoxazoles. Chem. Eur. J. 21, 18915–18920 (2015).
Lu, B., Wu, J. & Yoshikai, N. Palladium-catalyzed condensation of N-aryl imines and alkynylbenziodoxolones to form multisubstituted furans. J. Am. Chem. Soc. 136, 11598–11601 (2014).
Zhang, K.-F. et al. Nickel-catalyzed carbofluoroalkylation of 1,3-enynes to access structurally diverse fluoroalkylated allenes. Angew. Chem. Int. Ed. 58, 5069–5074 (2019).
Acknowledgements
This work was generously supported by the Alexander von Humboldt Foundation (H.-M.H.) and the Deutsche Forschungsgemeinschaft (Leibniz Award, SBF 858).
Author information
Authors and Affiliations
Contributions
H.-M.H. and P.B. equally contributed to the literature search and writing of the article, J.M. contributed to the preparation of figures, T.D. contributed to the editing of the manuscript and F.G. coordinated the project and supervised the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Photoredox chemistry
-
Approaches that rely on the ability of metal complexes and organic dyes to convert visible light into chemical energy by engaging in single-electron transfer with organic substrates, thereby generating reactive intermediates.
- Electrosynthesis
-
Synthesis of chemical compounds that exploits an electrochemical cell to facilitate redox processes.
- Atom transfer radical addition
-
(ATRA). Reaction class that involves radical atom transfer over a multiple-bond system (e.g. alkene), yielding bifunctionalized species with high atom-economy.
- Radical chain process
-
Chemical reaction that involves propagation of radical processes via the intermediacy of free radicals.
- Energy transfer
-
Transfer of energy between one excited (the photosensitizer) and one ground state species, via either Dexter or Förster-type energy transfer.
- Boron-dipyrromethene
-
(BODIPY). Heterocyclic organoboron class of compounds, investigated for its highly tunable optical properties.
- Single-electron transfer
-
(SET). Step in a chemical reaction characterized by donation or removal of an electron.
- Radical Truce–Smiles rearrangement
-
An intramolecular, nucleophilic ipso substitution on an aromatic ring system, activated by electron withdrawing group(s) at the ortho-position(s) and/or para-position(s) with respect to the reaction centre.
- Hydrogen atom transfer
-
(HAT). Concerted transfer of a proton and an electron to result overall in the movement of a hydrogen atom.
- 1,5-Hydrogen atom transfer
-
Intramolecular abstraction of a hydrogen atom from a radical species located five atoms from the reactive centre to affect the overall migration of radicals along a chain.
- Suzuki–Miyaura cross-coupling
-
Palladium-catalysed coupling reaction between a halide (often aryl or alkenyl) and an organoboron species (e.g. boronic acid, boronic ester) to forge a new C–C bond.
- Heck reaction
-
Palladium-catalysed coupling reaction between a halide (often aryl or alkenyl) and an olefin to forge a new C–C bond.
- Hypervalent iodine reagents
-
Species that contain an iodine atom either in its trivalent or in its pentavalent oxidation state; these species have found widespread application in oxidative processes and cross-coupling reactions.
- Ullmann coupling
-
Coupling reaction between two aryl halide species to yield biaryls, usually catalysed by a copper salt.
- Carbenoid
-
Reactive intermediate that possesses features resembling a carbene.
- ipso Substitution
-
Substitution reaction occurring in aromatic systems at the carbon bearing the leaving group, usually via a nucleophilic aromatic displacement; less frequently, an electrophilic aromatic substitution at the ipso carbon can be observed.
- Dearomatization
-
Process or elemental step that involves the loss of aromatic character.
- Organocatalysis
-
Branch of catalysis featuring an organic molecule as catalyst.
- N-heterocyclic carbene
-
(NHC). Molecular species featuring a ring structure and containing a divalent carbon atom bearing only six valence electrons, often used as ligand in transition-metal catalysis and organocatalysis.
- Ireland–Coates–Claisen rearrangement
-
[3+3]-Sigmatropic rearrangement of a silyl ketene acetal of an allyl ester to give a γ-β unsaturated carboxylic acid.
- Polymetallate species
-
Chemical species containing more than one metallic atom.
- Sonogashira coupling
-
Palladium-catalysed cross-coupling reaction between a halide (often aryl or alkenyl) and a terminal alkyne. Involves the in situ formation of copper acetylides that undergo transmetallation.
Rights and permissions
About this article
Cite this article
Huang, HM., Bellotti, P., Ma, J. et al. Bifunctional reagents in organic synthesis. Nat Rev Chem 5, 301–321 (2021). https://doi.org/10.1038/s41570-021-00266-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-021-00266-5
This article is cited by
-
Couple-close construction of polycyclic rings from diradicals
Nature (2024)
-
Photocatalytic vinyl radical-mediated multicomponent 1,4-/1,8-carboimination across alkynes and olefins/(hetero)arenes
Science China Chemistry (2024)
-
Photoredox catalytic alkylarylation of alkynes with arylsulfonylacetate as bifunctional reagent
Science China Chemistry (2024)
-
Modular access to alkylgermanes via reductive germylative alkylation of activated olefins under nickel catalysis
Nature Communications (2023)
-
Photoinduced difunctionalization with bifunctional reagents containing N-heteroaryl moieties
Science China Chemistry (2023)