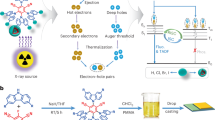
Abstract
X-ray detection, which plays an important role in medical and industrial fields, usually relies on inorganic scintillators to convert X-rays to visible photons; although several high-quantum-yield fluorescent molecules have been tested as scintillators, they are generally less efficient. High-energy radiation can ionize molecules and create secondary electrons and ions. As a result, a high fraction of triplet states is generated, which act as scintillation loss channels. Here we found that X-ray-induced triplet excitons can be exploited for emission through very rapid, thermally activated up-conversion. We report scintillators based on three thermally activated delayed fluorescence molecules with different emission bands, which showed significantly higher efficiency than conventional anthracene-based scintillators. X-ray imaging with 16.6 line pairs mm−1 resolution was also demonstrated. These results highlight the importance of efficient and prompt harvesting of triplet excitons for efficient X-ray scintillation and radiation detection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The source data related to the main figures and extended data figures are provided with this manuscript. Further data are available from the authors upon request. Source data are provided with this paper.
Change history
04 March 2022
A Correction to this paper has been published: https://doi.org/10.1038/s41563-022-01226-0
References
Tang, C. W. & VanSlyke, S. A. Organic electroluminescent diodes. Appl. Phys. Lett. 51, 913–915 (1987).
Burroughes, J. H. et al. Light-emitting diodes based on conjugated polymers. Nature 347, 539–541 (1990).
Baldo, M. A. et al. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 395, 151–154 (1998).
Adachi, C., Baldo, M. A., Forrest, S. R. & Thompson, M. E. High-efficiency organic electrophosphorescent devices with tris (2-phenylpyridine) iridium doped into electron-transporting materials. Appl. Phys. Lett. 77, 904–906 (2000).
Adachi, C., Baldo, M. A., Thompson, M. E. & Forrest, S. R. Nearly 100% internal phosphorescence efficiency in an organic light-emitting device. J. Appl. Phys. 90, 5048–5051 (2001).
Uoyama, H., Goushi, K., Shizu, K., Nomura, H. & Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 492, 234–238 (2012).
Zhang, Q. et al. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photon. 8, 326 (2014).
Ai, X. et al. Efficient radical-based light-emitting diodes with doublet emission. Nature 563, 536–540 (2018).
Di, D. et al. High-performance light-emitting diodes based on carbene-metal-amides. Science 356, 159–163 (2017).
Lamansky, S. et al. Highly phosphorescent bis-cyclometalated iridium complexes: synthesis, photophysical characterization, and use in organic light emitting diodes. J. Am. Chem. Soc. 123, 4304–4312 (2001).
Tang, C. W. Two‐layer organic photovoltaic cell. Appl. Phys. Lett. 48, 183–185 (1986).
Yu, G., Gao, J., Hummelen, J. C., Wudl, F. & Heeger, A. J. Polymer photovoltaic cells: enhanced efficiencies via a network of internal donor–acceptor heterojunctions. Science 270, 1789–1791 (1995).
Sariciftci, N. S., Smilowitz, L., Heeger, A. J. & Wudl, F. Photoinduced electron transfer from a conducting polymer to buckminsterfullerene. Science 258, 1474–1476 (1992).
Burlingame, Q. et al. Intrinsically stable organic solar cells under high-intensity illumination. Nature 573, 394–397 (2019).
Sirringhaus, H. et al. High-resolution inkjet printing of all-polymer transistor circuits. Science 290, 2123–2126 (2000).
Mykhaylyk, V. B., Kraus, H. & Saliba, M. Bright and fast scintillation of organolead perovskite MAPbBr3 at low temperatures. Mater. Horiz. 6, 1740–1747 (2019).
Wei, H. & Huang, J. Halide lead perovskites for ionizing radiation detection. Nat. Commun. 10, 1066 (2019).
Birks, J. B. Scintillations from organic crystals: specific fluorescence and relative response to different radiations. Proc. Phys. Soc. A 64, 874 (1951).
Sangster, R. C. & Irvine, J. W. Jr Study of organic scintillators. J. Chem. Phys. 24, 670–715 (1956).
Fraboni, B., Fraleoni‐Morgera, A. & Zaitseva, N. Ionizing radiation detectors based on solution‐grown organic single crystals. Adv. Funct. Mater. 26, 2276–2291 (2016).
Yanagida, T., Watanabe, K. & Fujimoto, Y. Comparative study of neutron and gamma-ray pulse shape discrimination of anthracene, stilbene, and p-terphenyl. Nucl. Instrum. Methods Phys. Res. A 784, 111–114 (2015).
Brooks, F. Development of organic scintillators. Nucl. Instrum. Methods 162, 477–505 (1979).
Maddalena, F. et al. Inorganic, organic, and perovskite halides with nanotechnology for high–light yield X- and γ-ray scintillators. Crystals 9, 88 (2019).
Chen, Q. et al. All-inorganic perovskite nanocrystal scintillators. Nature 561, 88–93 (2018).
Voltz, R., Dupont, H. & Laustriat, G. Radioluminescence des milieux organiques. II. Vérification expérimentale de l'étude cinétique. J. Phys. (Fr.) 29, 297–305 (1968).
Laustriat, G. The luminescence decay of organic scintillators. Mol. Cryst. 4, 127–145 (1968).
Fuchs, C., Klein, J. & Voltz, R. Magnetic-field-modulated and time-resolved luminescence in organic crystals excited by high energy radiation. Radiat. Phys. Chem. (1977) 21, 67–76 (1983).
Wen, Y. et al. Achieving highly efficient pure organic single-molecule white-light emitter: the coenhanced fluorescence and phosphorescence dual emission by tailoring alkoxy substituents. Adv. Opt. Mater. 8, 1901995 (2020).
Huang, R. et al. The contributions of molecular vibrations and higher triplet levels to the intersystem crossing mechanism in metal-free organic emitters. J. Mater. Chem. C 5, 6269–6280 (2017).
Hirata, S. et al. Effect of the tris(trimethylsilyl)silyl group on the fluorescence and triplet yields of oligothiophenes. J. Phys. Chem. C 124, 3277–3286 (2020).
Tsai, W.-L. et al. A versatile thermally activated delayed fluorescence emitter for both highly efficient doped and non-doped organic light emitting devices. Chem. Commun. 51, 13662–13665 (2015).
Seino, Y., Inomata, S., Sasabe, H., Pu, Y. J. & Kido, J. High‐performance green OLEDs using thermally activated delayed fluorescence with a power efficiency of over 100 lm W−1. Adv. Mater. 28, 2638–2643 (2016).
dos Santos, P. L., Etherington, M. K. & Monkman, A. P. Chemical and conformational control of the energy gaps involved in the thermally activated delayed fluorescence mechanism. J. Mater. Chem. C 6, 4842–4853 (2018).
Dias, F. B., Penfold, T. J. & Monkman, A. P. Photophysics of thermally activated delayed fluorescence molecules. Methods Appl. Fluoresc. 5, 012001 (2017).
Heo, J. H. et al. High‐performance next‐generation perovskite nanocrystal scintillator for nondestructive X‐ray imaging. Adv. Mater. 30, 1801743 (2018).
Yaffe, M. J. & Rowlands, J. A. X-ray detectors for digital radiography. Phys. Med. Biol. 42, 1–39 (1997).
Kang, Z. et al. CdTe quantum dots and polymer nanocomposites for X-ray scintillation and imaging. Appl. Phys. Lett. 98, 181914 (2011).
Wu, Y. et al. CsI:Tl+,Yb2+: ultra-high light yield scintillator with reduced afterglow. CrystEngComm 16, 3312–3317 (2014).
King, T. & Voltz, R. The time dependence of scintillation intensity in aromatic materials. Proc. R. Soc. Lond. A. Math. Phys. Sci. 289, 424–439 (1966).
Tang, X. et al. Efficient nondoped blue fluorescent organic light-emitting diodes (OLEDs) with a high external quantum efficiency of 9.4% @ 1000 cd m−2 based on phenanthroimidazole−anthracene derivative. Adv. Funct. Mater. 28, 1705813 (2018).
Büchele, P., Richter, M., Tedde, S. F., Matt, G. J. & Schmidt, O. X-ray imaging with scintillator-sensitized hybrid organic photodetectors. Nat. Photon. 9, 843 (2015).
Nobuyasu, R. S. et al. Rational design of TADF polymers using a donor–acceptor monomer with enhanced TADF efficiency induced by the energy alignment of charge transfer and local triplet excited states. Adv. Opt. Mater. 4, 597–607 (2016).
Schinabeck, A. et al. Symmetry-based design strategy for unprecedentedly fast decaying thermally activated delayed fluorescence (TADF). Application to dinuclear Cu(i) compounds. Chem. Mater. 31, 4392–4404 (2019).
Gu, L. et al. Colour-tunable ultra-long organic phosphorescence of a single-component molecular crystal. Nat. Photon. 13, 406–411 (2019).
Hajagos, T. J., Liu, C., Cherepy, N. J. & Pei, Q. High‐Z sensitized plastic scintillators: a review. Adv. Mater. 30, 1706956 (2018).
O’Neal, S., Cherepy, N., Hok, S. & Payne, S. Performance of high stopping power bismuth-loaded plastic scintillators for radiation portal monitors. IEEE Trans. Nucl. Sci. 67, 746–751 (2020).
Acknowledgements
We thank S. Deng and S. Yao of Hamamatsu Photonics for their assistance with X-ray-related measurements and useful discussions, and B. Yang and H. Liu of Jilin University and Y. Zhang of Huzhou Normal University for assistance with material preparations. Y.(M.)Y. acknowledges funds received from the National Key Research and Development Program of China (2017YFA0207700), the Outstanding Youth Fund of Zhejiang Natural Science Foundation of China (LR18F050001) and the Natural Science Foundation of China (62074136, 61804134, 61874096).
Author information
Authors and Affiliations
Contributions
Y.(M.)Y. conceived the idea and designed the experiments. W.M. and Y.S. prepared the scintillator molecules, characterized the performance and conducted the X-ray-related experiments. W.M. derived the mathematical model to quantify the X-ray-induced photoexcitation. Y.(M.)Y., W.M. and Y.S. analysed the results. Q.Z. and C.D. provided insightful discussions on the photophysics and chemistry of the TADF mechanism and provided some TADF molecules for the study. L.P. provided insightful discussions on the mechanism of X-ray interactions with organic molecules. C.D., Z.C. and H.Zhu. helped with lifetime measurements, and participated in helpful discussions. P.H., W.Z., Y.S. and H.Zhong. synthesized the CsPbBr3 NCs. Y.T., P.R. and T.L. assisted with X-ray imaging measurements. G.Y., G.L., K.W., S.P. and J.X. helped with the transient absorption measurements. X.L. and H.L. provided insightful discussions on optical system design. W.M. plotted the data and prepared the figures. Y.(M.)Y. wrote the manuscript with input from W.M. and Y.S.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Materials thanks Beatrice Fraboni and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Measurement set-up of the RL intensities of organic scintillators.
Schematic of the testing system for radioluminescence (RL) intensity measurement.
Extended Data Fig. 2 HOMO and LUMO of organic scintillators.
Highest occupied molecular orbitals (HOMO) and lowest unoccupied molecular orbitals (LUMO) according to the results of time-dependent DFT for the S1 states of Anthracene, DMAc-TRZ, 4CzIPN and 4CzTPN-Bu using the optimized structure of the S0 states. Colors indicate the different phases of the natural transition orbitals.
Extended Data Fig. 3 Normalized RL spectra of organic scintillators when thickness increasing.
Normalized RL spectra at the thicknesses of 0.1, 0.2, 0.4 and 0.8 mm of a, Anthracene, b, DMAc-TRZ, c, 4CzIPN and d, 4CzTPN-Bu, respectively. (X-ray tube voltage: 50 kV; dose rate: 3.034 mGyair s-1).
Extended Data Fig. 4 Normalized PL and PLE spectra of organic scintillators.
The normalized photoluminescence (PL, solid lines) spectra and the normalized photoluminescence excitation (PLE, dotted lines) spectra of DMAc-TRZ, 4CzIPN and 4CzTPN-Bu in toluene (left) compared with that of Anthracene in toluene (right).
Extended Data Fig. 5 X-ray attenuation efficiency spectra of organic scintillators and output spectra of X-ray tube.
a, Output spectra at different tube voltages of Mini-X X-ray tube (target: Ag, characteristic peak is at 22 keV). b, Calculated X-ray attenuation efficiencies of Anthracene, DMAc-TRZ, 4CzIPN, 4CzTPN-Bu and sucrose octaacetate (SO) versus thicknesses of these scintillators. c, Calculated X-ray attenuation coefficient (cm-1) spectra of Anthracene, DMAc-TRZ, 4CzIPN, 4CzTPN-Bu and SO, respectively. d, Calculated X-ray attenuation efficiency spectra of Anthracene, DMAc-TRZ, 4CzIPN, 4CzTPN-Bu and SO at thickness of 400 μm (the thickness value for calculation of light yield).
Extended Data Fig. 6 RL intensity spectra of the diluted organic scintillator screens.
RL intensity spectra of 10 wt% Anthracene:SO screen, 10 wt% DMAc-TRZ:SO screen, 10 wt% 4CzIPN:SO screen and 10 wt% 4CzTPN-Bu:SO screen.
Extended Data Fig. 7 RL linear relationships of TADF organic scintillators.
The RL intensities versus biggish X-ray dose rates of DMAc-TRZ, 4CzIPN and 4CzTPN-Bu, respectively. The error bars were determined by the measurements of three samples and each sample was measured three times. It can be obviously observed that these TADF organic scintillators show good linear relationships between RL intensity and X-ray dose rate. (X-ray tube voltage: 50 kV, current: 79 μA).
Extended Data Fig. 8 Photographs and X-ray images of TADF organic scintillator screens.
a-c, Photographs under a, bright field, b, ultraviolet illumination and c, X-ray irradiation of 10 wt% DMAc-TRZ:SO scintillator screen, 10 wt% 4CzIPN:SO scintillator screen and 10 wt% 4CzTPN-Bu:SO scintillator screen, respectively. d, X-ray images of encapsulated metallic spring collected by 10 wt% DMAc-TRZ:SO scintillator screen, 10 wt% 4CzIPN:SO scintillator screen and 10 wt% 4CzTPN-Bu:SO scintillator screen, respectively. (X-ray tube voltage: 50 kV; dose rate: 75.5 μGyair s-1).
Supplementary information
Supplementary Information
Supplementary Discussions 1–3, Figs. 1–11 and Tables 1–5.
Supplementary Video 1
Comparison of RL performances among three TADF scintillators and anthracene.
Supplementary Video 2
PL and RL performances of CsPbBr3 QDs and DMAc-TRZ.
Source data
Source Data Fig. 2
Source Data for Figs. 2a,2b and 2d.
Source Data Fig. 3
Source Data for Fig. 3.
Source Data Fig. 4
Source Data for Fig. 4c.
Source Data Extended Data Fig. 3
Source Data for Extended Data Fig. 3.
Source Data Extended Data Fig. 4
Source Data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Source Data for Extended Data Fig. 5b-d.
Source Data Extended Data Fig. 6
Source Data for Extended Data Fig. 6.
Source Data Extended Data Fig. 7
Source Data for Extended Data Fig. 7.
Rights and permissions
About this article
Cite this article
Ma, W., Su, Y., Zhang, Q. et al. Thermally activated delayed fluorescence (TADF) organic molecules for efficient X-ray scintillation and imaging. Nat. Mater. 21, 210–216 (2022). https://doi.org/10.1038/s41563-021-01132-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41563-021-01132-x
This article is cited by
-
Efficient and ultrafast organic scintillators by hot exciton manipulation
Nature Photonics (2024)
-
X-ray-activated polymerization expanding the frontiers of deep-tissue hydrogel formation
Nature Communications (2024)
-
Water-dispersible X-ray scintillators enabling coating and blending with polymer materials for multiple applications
Nature Communications (2024)
-
Electron beam-induced white emission from iridium complexes-doped polymer dots
Photochemical & Photobiological Sciences (2024)
-
Efficient X-ray luminescence imaging with ultrastable and eco-friendly copper(I)-iodide cluster microcubes
Light: Science & Applications (2023)