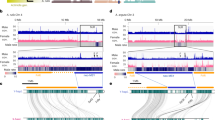
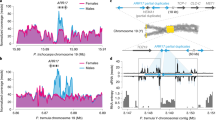
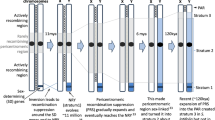
Abstract
Hundreds of land plant lineages have independently evolved separate sexes in either gametophytes (dioicy) or sporophytes (dioecy), but 43% of all dioecious angiosperms are found in just 34 entirely dioecious clades, suggesting that their mode of sex determination evolved a long time ago. Here, we review recent insights on the molecular mechanisms that underlie the evolutionary change from individuals that each produce male and female gametes to individuals specializing in the production of just one type of gamete. The canonical model of sex chromosome evolution in plants predicts that two sex-determining genes will become linked in a sex-determining region (SDR), followed by expanding recombination suppression, chromosome differentiation and, ultimately, degeneration. Experimental work, however, is showing that single genes function as master regulators in model systems, such as the liverwort Marchantia and the angiosperms Diospyros and Populus. In Populus, this type of regulatory function has been demonstrated by genome editing. In other systems, including Actinidia, Asparagus and Vitis, two coinherited factors appear to independently regulate female and male function, yet sex chromosome differentiation has remained low. We discuss the best-understood systems and evolutionary pathways to dioecy, and present a meta-analysis of the sizes and ages of SDRs. We propose that limited sexual conflict explains why most SDRs are small and sex chromosomes remain homomorphic. It appears that models of increasing recombination suppression with age do not apply because selection favours mechanisms in which sex determination depends on minimal differences, keeping it surgically precise.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Darwin, C. The Effects of Cross and Self Fertilisation in the Vegetable Kingdom (John Murray, 1876).
Renner, S. S. The relative and absolute frequencies of angiosperm sexual systems: dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 101, 1588–1596 (2014).
Charlesworth, B. & Charlesworth, D. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112, 975–997 (1978).
Henry, I. M., Akagi, T., Tao, R. & Comai, L. One hundred ways to invent the sexes: theoretical and observed paths to dioecy in plants. Annu. Rev. Plant Biol. 69, 553–575 (2018).
Akagi, T., Henry, I. M., Tao, R. & Comai, L. A Y-chromosome-encoded small RNA acts as a sex determinant in persimmons. Science 346, 646–650 (2014).
Charlesworth, D. Plant contributions to our understanding of sex chromosome evolution. New Phytol. 208, 52–65 (2015).
Renner, S. S. Pathways for making unisexual flowers and unisexual plants: moving beyond the “two mutations linked on one chromosome” model. Am. J. Bot. 103, 587–589 (2016).
Müller, N. A. et al. A single gene underlies the dynamic evolution of poplar sex determination. Nat. Plants 6, 630–637 (2020).
Cronk, Q. & Müller, N. A. Default sex and single gene sex determination in dioecious plants. Front. Plant Sci. 11, 1162 (2020).
Golenberg, E. M. & West, N. W. Hormonal interactions and gene regulation can link monoecy and environmental plasticity to the evolution of dioecy in plants. Am. J. Bot. 100, 1022–1037 (2013).
Yang, H.-W., Akagi, T., Kawakatsu, T. & Tao, R. Gene networks orchestrated by MeGI: a single-factor mechanism underlying sex determination in persimmon. Plant J. 98, 97–111 (2019).
Cronk, Q., Soolanayakanahally, R. & Brautigam, K. Gene expression trajectories during male and female reproductive development in balsam poplar (Populus balsamifera L.). Sci. Rep. 10, 8413 (2020).
McKown, A. D. et al. Sexual homomorphism in dioecious trees: extensive tests fail to detect sexual dimorphism in Populus. Sci. Rep. 7, 1831 (2017).
Charlesworth, D., Charlesworth, B. & Marais, G. Steps in the evolution of heteromorphic sex chromosomes. Heredity 95, 118–128 (2005).
Charlesworth, D. Plant sex chromosome evolution. J. Exp. Bot. 64, 405–420 (2013).
Charlesworth, D. Plant sex chromosomes. Annu. Rev. Plant Biol. 67, 397–420 (2016).
Charlesworth, D. Plant sex determination and sex chromosomes. Heredity 88, 94–101 (2002).
Charlesworth, D. Plant evolution: modern sex chromosomes. Curr. Biol. 14, R271–R273 (2004).
Charlesworth, D. Young sex chromosomes in plants and animals. New Phytol. 224, 1095–1107 (2019).
Janousek, B. & Mrackova, M. Sex chromosomes and sex determination pathway dynamics in plant and animal models. Biol. J. Linn. Soc. 100, 737–752 (2010).
Hobza, R. et al. Sex and the flower—developmental aspects of sex chromosome evolution. Ann. Bot. 122, 1085–1101 (2018).
Bateman, R. M. & DiMichele, W. A. Heterospory: The most iterative key innovation in the evolutionary history of the plant kingdom. Biol. Rev. 69, 345–417 (1994).
Friis, E. M., Crane, P. R. & Pedersen, K. R. Early Flowers and Angiosperm Evolution (Cambridge Univ. Press, 2011).
Käfer, J. et al. A derived ZW chromosome system in Amborella trichopoda, the sister species to all other extant flowering plants. Preprint at bioRxiv https://doi.org/10.1101/2020.12.21.423833 (2020).
Marks, R. A., Smith, J. J., Cronk, Q., Grassa, C. J. & McLetchie, D. N. Genome of the tropical plant Marchantia inflexa: implications for sex chromosome evolution and dehydration tolerance. Sci. Rep. 9, 8722 (2019).
Hisanaga, T. et al. A cis-acting bidirectional transcription switch controls sexual dimorphism in the liverwort. EMBO J. 38, e100240 (2019).
Eckenwalder, J. E. Systematics and Evolution of Populus (NRC Research Press, 1996).
Percy, D. M. et al. Understanding the spectacular failure of DNA barcoding in willows (Salix): does this result from aÿtrans-specific selective sweep? Mol. Ecol. 23, 4737–4756 (2014).
Manchester, S. R., Judd, W. S. & Handley, B. Foliage and fruits of early poplars (Salicaceae: Populus) from the eocene of Utah, Colorado, and Wyoming. Int. J. Plant Sci. 167, 897–908 (2006).
Sanderson, B. J. et al. Sex determination through X–Y heterogamety in Salix nigra. Heredity https://doi.org/10.1038/s41437-020-00397-3 (2021).
Almeida, P. et al. Genome assembly of the basket willow, Salix viminalis, reveals earliest stages of sex chromosome expansion. BMC Biol. 18, 78 (2020).
Zhou, R. et al. A willow sex chromosome reveals convergent evolution of complex palindromic repeats. Genome Biol. 21, 38 (2020).
Zhang, Z. et al. Improved genome assembly provides new insights into genome evolution in a desert poplar (Populus euphratica). Mol. Ecol. Resour. 20, 781–794 (2020).
Schiffthaler, B. et al. An improved genome assembly of the European aspen Populus tremula. Preprint at bioRxiv https://doi.org/10.1101/805614 (2019).
Tuskan, G. A. et al. The genome of black cottonwood, Populus trichocarpa (Torr. & Gray). Science 313, 1596–1604 (2006).
Wei, S., Yang, Y. & Yin, T. The chromosome-scale assembly of the willow genome provides insight into Salicaceae genome evolution. Horticulture Res. 7, 45 (2020).
Geraldes, A. et al. Recent Y chromosome divergence despite ancient origin of dioecy in poplars (Populus). Mol. Ecol. 24, 3243–3256 (2015).
Akagi, T., Henry, I. M., Kawai, T., Comai, L. & Tao, R. Epigenetic regulation of the sex determination gene MeGI in polyploid persimmon. Plant Cell 28, 2905–2915 (2016).
Akagi, T. et al. The persimmon genome reveals clues to the evolution of a lineage-specific sex determination system in plants. PLoS Genet. 16, e1008566 (2020).
Suo, Y. et al. A high-quality chromosomal genome assembly of Diospyros oleifera Cheng. Gigascience 9, giz164.
Fujito, S. et al. Evidence for a common origin of homomorphic and heteromorphic sex chromosomes in distinct Spinacia species. G3 5, 1663–1673 (2015).
Yu, L., Ma, X., Deng, B., Yue, J. & Ming, R. Construction of high-density genetic maps defined sex determination region of the Y chromosome in spinach. Mol. Genet. 296, 41–53 (2020).
Okazaki, Y., Takahata, S., Hirakawa, H., Suzuki, Y. & Onodera, Y. Molecular evidence for recent divergence of X- and Y-linked gene pairs in Spinacia oleracea L. PLoS ONE 14, e0214949 (2019).
West, N. W. & Golenberg, E. M. Gender-specific expression of GIBBERELLIC ACID INSENSITIVE is critical for unisexual organ initiation in dioecious Spinacia oleracea. New Phytol. 217, 1322–1334 (2018).
Huang, S. et al. Draft genome of the kiwifruit Actinidia chinensis. Nat. Commun. 4, 2640 (2013).
Pilkington, S. M. et al. A manually annotated Actinidia chinensis var. chinensis (kiwifruit) genome highlights the challenges associated with draft genomes and gene prediction in plants. BMC Genomics 19, 257 (2018).
Wu, H. et al. A high-quality Actinidia chinensis (kiwifruit) genome. Horticulture Res. 6, 117 (2019).
Tang, W. et al. Chromosome-scale genome assembly of kiwifruit Actinidia eriantha with single-molecule sequencing and chromatin interaction mapping. Gigascience 8, giz027 (2019).
Akagi, T. et al. A Y-encoded suppressor of feminization arose via lineage-specific duplication of a cytokinin response regulator in kiwifruit. Plant Cell 30, 780–795 (2018).
Akagi, T., Pilkington, S. M., Varkonyi-Gasic, E. & Henry, I. M. Two Y-chromosome-encoded genes determine sex in kiwifruit. Nat. Plants 5, 801–809 (2019).
Norup, M. F. et al. Evolution of Asparagus L. (Asparagaceae): out-of-South-Africa and multiple origins of sexual dimorphism. Mol. Phylogenet. Evol. 92, 25–44 (2015).
Harkess, A. et al. The asparagus genome sheds light on the origin and evolution of a young Y chromosome. Nat. Commun. 8, 1279 (2017).
Harkess, A. et al. Sex determination by two Y-linked genes in garden asparagus. Plant Cell 32, 1790–1796 (2020).
Li, S.-F. et al. Chromosome-level genome assembly, annotation and evolutionary analysis of the ornamental plant Asparagus setaceus. Horticulture Res. 7, 48 (2020).
Deng, C. L. et al. Karyotype of asparagus by physical mapping of 45S and 5S rDNA by FISH. J. Genet. 91, 209–212 (2012).
Casimiro-Soriguer, I., Buide, M. L. & Narbona, E. Diversity of sexual systems within different lineages of the genus Silene. AoB PLANTS 7, plv037 (2015).
Slancarova, V. et al. Evolution of sex determination systems with heterogametic males and females in Silene. Evolution 67, 3669–3677 (2013).
Howell, E. C., Armstrong, S. J. & Filatov, D. A. Evolution of neo-sex chromosomes in Silene diclinis. Genetics 182, 1109–1115 (2009).
Balounova, V. et al. Evolution of sex determination and heterogamety changes in section Otites of the genus Silene. Sci. Rep. 9, 1045 (2019).
Martin, H. et al. Evolution of young sex chromosomes in two dioecious sister plant species with distinct sex determination systems. Genome Biol. Evol. 11, 350–361 (2019).
Barankova, S. et al. Sex-chrom, a database on plant sex chromosomes. New Phytol. 227, 1594–1604 (2020).
Westergaard, M. Aberrant Y chromosomes and sex expression in Melandrium album. Hereditas 32, 419–443 (1946).
Kazama, Y. et al. A new physical mapping approach refines the sex-determining gene positions on the Silene latifolia Y-chromosome. Sci. Rep. 6, 18917 (2016).
Hazzouri, K. M. et al. Genome-wide association mapping of date palm fruit traits. Nat. Commun. 10, 4680 (2019).
Torres, M. F. et al. Genus-wide sequencing supports a two-locus model for sex-determination in Phoenix. Nat. Commun. 9, 3969 (2018).
Carvalho, F. A. & Renner, S. S. A dated phylogeny of the papaya family (Caricaceae) reveals the crop’s closest relatives and the family’s biogeographic history. Mol. Phylogenet. Evol. 65, 46–53 (2012).
Wang, J. et al. Sequencing papaya X and Yh chromosomes reveals molecular basis of incipient sex chromosome evolution. Proc. Natl Acad. Sci. USA 109, 13710–13715 (2012).
VanBuren, R. et al. Origin and domestication of papaya Yh chromosome. Genome Res. 25, 524–533 (2015).
Picq, S. et al. A small XY chromosomal region explains sex determination in wild dioecious V. vinifera and the reversal to hermaphroditism in domesticated grapevines. BMC Plant Biol. 14, 229 (2014).
Zhou, Y., Massonnet, M., Sanjak, J. S., Cantu, D. & Gaut, B. S. Evolutionary genomics of grape (Vitis vinifera ssp. vinifera) domestication. Proc. Natl Acad. Sci. USA 114, 11715–11720 (2017).
Fechter, I. et al. Candidate genes within a 143 kb region of the flower sex locus in Vitis. Mol. Genet Genomics 287, 247–259 (2012).
Coito, J. L. et al. VviAPRT3 and VviFSEX: two genes involved in sex specification able to distinguish different flower types in Vitis. Front. Plant Sci. 8, 98 (2017).
Massonnet, M. et al. The genetic basis of sex determination in grapes. Nat. Commun. 11, 2902 (2020).
Badouin, H. et al. The wild grape genome sequence provides insights into the transition from dioecy to hermaphroditism during grape domestication. Genome Biol. 21, 223 (2020).
Ahmadi, H. & Bringhurst, R. S. Genetics of sex expression in Fragaria species. Am. J. Bot. 78, 504–514 (1991).
Shulaev, V. et al. The genome of woodland strawberry (Fragaria vesca). Nat. Genet. 43, 109–116 (2011).
Edger, P. P. et al. Single-molecule sequencing and optical mapping yields an improved genome of woodland strawberry (Fragaria vesca) with chromosome-scale contiguity. Gigascience 7, gix124 (2018).
Edger, P. P. et al. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 51, 541–547 (2019).
Tennessen, J. A. et al. Repeated translocation of a gene cassette drives sex-chromosome turnover in strawberries. PLoS Biol. 16, e2006062 (2018).
Ashman, T.-L. et al. Multilocus sex determination revealed in two populations of gynodioecious wild strawberry, Fragaria vesca subsp. bracteata. G3 5, 2759 (2015).
Kamiya, T. et al. A trans-species missense SNP in Amhr2 is associated with sex determination in the tiger pufferfish, Takifugu rubripes (fugu). PLoS Genet. 8, e1002798 (2012).
Ming, R., Bendahmane, A. & Renner, S. S. Sex chromosomes in land plants. Annu Rev. Plant Biol. 62, 485–514 (2011).
Sousa, A., Fuchs, J. & Renner, S. S. Molecular cytogenetics (FISH, GISH) of Coccinia grandis: a ca. 3 myr-old species of cucurbitaceae with the largest Y/autosome divergence in flowering plants. Cytogenet. Genome Res. 139, 107–118 (2013).
Sousa, A., Fuchs, J. & Renner, S. S. Cytogenetic comparison of heteromorphic and homomorphic sex chromosomes in Coccinia (Cucurbitaceae) points to sex chromosome turnover. Chromosome Res. 25, 191–200 (2017).
Perrin, N. Sex reversal: a fountain of youth for sex chromosomes? Evolution 63, 3043–3049 (2009).
Roco, A. S. et al. Coexistence of Y, W, and Z sex chromosomes in Xenopus tropicalis. Proc. Natl Acad. Sci. USA 112, E4752–E4761 (2015).
Charlesworth, B. The evolution of sex chromosomes. Science 251, 1030–1033 (1991).
Immler, S. & Otto, S. P. The evolution of sex chromosomes in organisms with separate haploid sexes. Evolution 69, 694–708 (2015).
Papadopulos, A. S., Chester, M., Ridout, K. & Filatov, D. A. Rapid Y degeneration and dosage compensation in plant sex chromosomes. Proc. Natl Acad. Sci. USA 112, 13021–13026 (2015).
Prentout, D. et al. An efficient RNA-seq-based segregation analysis identifies the sex chromosomes of Cannabis sativa. Genome Res. 30, 164–172 (2020).
Darwin, C. The Different Forms of Flowers on Plants of the Same Species (John Murray, 1977).
Godin, V. N. & Demyanova, E. I. On the distribution of gynodioecy in flowering plants. Botanicheskiy Zh. 93, 1465–1487 (2013).
Renner, S. S. & Ricklefs, R. E. Dioecy and its correlates in the flowering plants. Am. J. Bot. 82, 596–606 (1995).
Frank, S. A. The evolutionary dynamics of cytoplasmic male sterility. Am. Nat. 133, 345–376 (1989).
McCauley, D. E. & Bailey, M. F. Recent advances in the study of gynodioecy: the interface of theory and empiricism. Ann. Bot. 104, 611–620 (2009).
Spigler, R. B. & Ashman, T.-L. Gynodioecy to dioecy: are we there yet? Ann. Bot. 109, 531–543 (2011).
Westergaard, M. The mechanism of sex determination in dioecious flowering plants. Adv. Genet. 9, 217–281 (1958).
Lloyd, D. G. Breeding systems in Cotula L. (Compositae, Anthemideae). I. The array of monoclinous and diclinous systems. New Phytol. 71, 1181–1194 (1972).
Lloyd, D. G. Breeding systems in Cotula L. (Compositae, Anthemideae). II. Monoecious populations. New Phytol. 71, 1195–1202 (1972).
Lloyd, D. G. Breeding systems in Cotula. III. Dioecious populations. New Phytol. 74, 109–123 (1975).
Renner, S. S. & Won, H. Repeated evolution of dioecy from monoecy in Siparunaceae (Laurales). Syst. Biol. 50, 700–712 (2001).
Anger, N., Fogliani, B., Scutt, C. P. & Gateble, G. Dioecy in Amborella trichopoda: evidence for genetically based sex determination and its consequences for inferences of the breeding system in early angiosperms. Ann. Bot. 119, 591–597 (2017).
Jones, D. F. Unisexual maize plants and their bearing on sex differentiation in other plants and in animals. Genetics 19, 552–567 (1934).
Boualem, A. et al. A cucurbit androecy gene reveals how unisexual flowers develop and dioecy emerges. Science 350, 688–691 (2015).
Yin, T. & Quinn, J. A. Tests of a mechanistic model of one hormone regulating both sexes in Cucumis sativus (Cucurbitaceae). Am. J. Bot. 82, 1537–1546 (1995).
Lester, D. T. Variation in sex expression in Populus tremuloides Michx. Silvae Genet. 12, 141–151 (1963).
Cronk, Q. C. B., Needham, I. & Rudall, P. J. Evolution of catkins: inflorescence morphology of selected Salicaceae in an evolutionary and developmental context. Front. Plant Sci. 6, 1030 (2015).
Akagi, T. & Charlesworth, D. Pleiotropic effects of sex-determining genes in the evolution of dioecy in two plant species. Proc. R. Soc. B 286, 20191805 (2019).
Wang, M. et al. Phylogenomics of the genus Populus reveals extensive interspecific gene flow and balancing selection. New Phytol. 225, 1370–1382 (2020).
Shang, H. et al. Evolution of strong reproductive isolation in plants: broad-scale patterns and lessons from a perennial model group. Philos. Trans. R. Soc. Lond. B 375, 20190544 (2020).
Yang, W. et al. A general model to explain repeated turnovers of sex determination in the Salicaceae. Mol. Biol. Evol. 38, 968–980 (2021).
Acknowledgements
N.A.M. acknowledges funding from a grant from the German Research Foundation (DFG MU 4357/1-1) and S.S.R. is grateful for support from the Elfriede and Franz Jakob Foundation for research in the Botanical Garden Munich.
Author information
Authors and Affiliations
Contributions
S.S.R. and N.A.M. both performed literature searches and wrote the paper. N.A.M. produced the figures.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Plants thanks Takashi Akagi and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Fig. 1, Note 1 and references.
Supplementary Table 1
Information on plant SDRs, including gene number, physical size and age estimates.
Rights and permissions
About this article
Cite this article
Renner, S.S., Müller, N.A. Plant sex chromosomes defy evolutionary models of expanding recombination suppression and genetic degeneration. Nat. Plants 7, 392–402 (2021). https://doi.org/10.1038/s41477-021-00884-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41477-021-00884-3
This article is cited by
-
Genomic profiling of dioecious Amaranthus species provides novel insights into species relatedness and sex genes
BMC Biology (2023)
-
The male-heterogametic sex determination system on chromosome 15 of Salix triandra and Salix arbutifolia reveals ancestral male heterogamety and subsequent turnover events in the genus Salix
Heredity (2023)
-
Subgenome dominance shapes novel gene evolution in the decaploid pitcher plant Nepenthes gracilis
Nature Plants (2023)
-
Evolution of a ZW sex chromosome system in willows
Nature Communications (2023)
-
Sex-chrom v. 2.0: a database of green plant species with sex chromosomes
Chromosoma (2023)