Abstract
Background
The fetal brain starts developing early and animal studies have suggested that iron plays several roles for the development, but results from epidemiological studies investigating associations between gestational iron and offspring neurodevelopment are inconsistent.
Objective
To systematically examine results from observational studies and RCTs on gestational iron and offspring neurodevelopment, with focus on the importance of four domains: iron status indicators, exposure timing, neurodevelopmental outcomes, and offspring age.
Methods
PRISMA guidelines were followed. Embase, PsychInfo, Scopus, and The Cochrane library were searched in September 2017 and February 2018. Overall, 3307 articles were identified and 108 retrieved for full-text assessment. Pre-specified eligibility criteria were used to select studies and 27 articles were included;19 observational and 8 RCTs.
Results
Iron status in pregnancy was associated with offspring behavior, cognition, and academic achievement. The direction of associations with behavioral outcomes were unclear and the conclusions related to cognition and academic achievement were based on few studies, only. Little evidence was found for associations with motor development. Observed associations were shown to persist beyond infancy into adolescence, and results depended on iron status indicator type but not on the timing of exposure.
Conclusion
We conclude that there is some evidence that low pregnancy iron, possibly particularly in the 3rd trimester, may be associated with adverse offspring neurodevelopment. As most previous research used Hemoglobin, inferring results to iron deficiency should be done with caution. No conclusions could be reached regarding associations beyond early childhood, and supplementation with iron during pregnancy did not seem to influence offspring neurodevelopment.
Similar content being viewed by others
Introduction
Worldwide, iron deficiency (ID) is the most common micronutrient deficiency. Global data on ID in pregnancy is non-existent, however, using anemia as an indirect indicator, ID would seem to be prevalent both in developed and developing countries [1]. Though, the prevalence is higher in developing countries [2,3,4], especially in the Indian subcontinent and in sub-Saharan Africa, where according to the World Health Organization (WHO), up to 90% of women become anemic during pregnancy [5].
ID can affect the functions of the fetal developing brain and influence normal neurodevelopment later in life due to its biological roles in the fetal brain [6,7,8,9,10,11,12,13,14]. Iron is involved in the neurotransmitter systems [15,16,17] in gene expression and DNA synthesis [10, 17,18,19] and in energy metabolism [17, 20]. Iron also plays a protective role for brain development through the regulation of the neurotrophic factors [11] and is responsible for the myelination process of neurons [21]. However, iron overload can also cause pathological effects [12, 17] by producing oxygen reactive species [12, 22] that may adversely influence brain development.
Despite these proposed biological roles of prenatal iron, two recent reviews of results from human epidemiological studies relating prenatal iron exposure to offspring neurodevelopment conclude either that there are associations of ID with several neurodevelopmental outcomes [23], or that the evidence is insufficient [24]. However, a deeper examination of the potential reasons behind the inconsistent results has not previously been provided, but may be linked to differential results for (1) the different neurodevelopmental outcomes, (2) the different ages when outcomes have been assessed, (3) the different indicators used for assessing iron exposure or 4) the timing during pregnancy for assessing iron exposure.
Thus, in this review, we systematically review published observational studies and randormized controlled trials (RCTs) that examined relations between iron status during pregnancy and later neurodevelopment in the offspring and examine how study results may depend on the four above mentioned domains. Our findings are expected to give specific direction for future research and what needs to be prioritized to cover current gaps in knowledge.
Methods
This systematical literature review was registered on PROSPERO (CRD42017078312), and follows the PRISMA checklist (Supplemental file 1).
Search strategy and literature identification
We conducted literature searches in September 2017 and February 2018 using the following four electronic databases: Embase on Ovid, PsychInfo on EBSCO, Scopus, and The Cochrane library to identify all publications providing data on the associations between iron status in pregnancy and neurodevelopment of the offspring. No restrictions were applied on language, year of publication, country or study design. Reference lists of retrieved publications were reviewed to identify studies not obtained in the systematic search. Table 1 presents the terms used for searching all four databases.
Study selection
Initially, 3307 relevant articles were identified on the basis of titles and abstracts. These were scanned and excluded due to the following: (1) they did not investigate associations between iron status in pregnancy and offspring neurodevelopment; (2) were review articles, case reports or book chapters; (3) were animal studies or (4) were not in English. Where the title or abstract of an article did not have sufficient information for exclusion, the article’s full-text was assessed. We also hand-searched reference lists of retrieved articles and searched grey literature which led to the identification of 3 extra articles. Thus, full-texts of 108 articles were assessed.
Eligibility criteria
Pre-specified eligibility criteria were set using the PICOS tool [25]. The population in the included studies was pregnant women and their offspring, with no restriction on offspring age. In observational studies, blood iron biomarkers were measured and for RCTs, the intervention was initiated during pregnancy and/or preconception and was either iron alone or with another micronutrient and the comparator was either placebo or the micronutrient alone, respectively. We excluded studies of high-risk pregnancies; where the population was with chronic disease and psycho-neurologic disorders to allow for assessments in the absence of disease and to limit recall biases and studies relying on iron from self-reported diet intake were also excluded. The primary outcome was offspring neurodevelopment assessed using developmental assessment and screening tools. Only original observational studies and RCT articles were included.
Quality assessment and data extraction
Three different reviewers examined the articles to minimize errors and selection bias. First, data from included studies were extracted by one reviewer (JJ) using a modified form of the Cochrane data collection form (Supplemental file 2). Prior to data extraction, the form was pilot tested by extracting information from 2 randomly selected observational studies and 2 RCTs. Second, a second independent reviewer (MS) extracted the information from the same studies using the PICOS tool, and the information was then compared between the two reviewers to minimize errors. Observed differences in the extracted information were subsequently discussed and agreed on. In studies were several outcomes and exposures were investigated, only those that met the set eligibility criteria were extracted and included in this review. Third, the same two reviewers conducted a quality assessment of the included studies at study level independently and were blinded to each other’s assessments using the modified Downs & Black checklist (Supplemental file 3) [26]. Based on the scores resulting from assessing all included studies, we created three quality categories for observational studies: low (10–14 points), medium (15–19 points), and high quality (≥20 points) and for the RCTs: low (20–22 points), medium (23–25 points), and high quality (26–28 points). Discrepancies in scoring were resolved by discussion and if scores were not agreed on, a third reviewer (BLH) was brought in to resolve disagreements. Assessment of bias across studies was not performed because our review focused was on exploring the study domains which may or may not introduce such biases. RCTs were assessed on all items with a maximal score of 28 and observational studies were assessed on all but 4 items which were not applicable for observational designs and, thus, could get a maximal score of 24.
Presentation of results and categorization of domains
Results from all included studies were categorized according to the following four domains: (1) Iron status indicator, (2) Exposure timing, (3) Type of neurodevelopmental outcome, and (4) Offspring age at assessment of neurodevelopmental outcome.
Iron status indicator
Eleven categories were used: serum ferritin (SF), serum iron (SI), iron binding capacity (IBC), transferrin saturation (TS), zinc protoporphyrin (ZPP), serum transferrin receptor (sTfR), a combination of transferrin saturation and serum ferritin (TS + SF), a combination of serum ferritin, transferrin saturation, and mean corpuscular volume (TS + SF + MCV), a combination of hemoglobin and ferritin (Hb + SF), hemoglobin (Hb), and hematocrit (Ht). If there were no studies in one of the categories, that category was not represented.
Exposure timing
We used five main categories based on pregnancy trimesters’ definition of the National Institute of Health, Library of medicine on the PubMed database [27]. These were: The preconception period, the 1st, the 2nd, the 3rd trimester, and at delivery/birth. In studies where iron exposure did not fit into these categories, the reported mean or median pregnancy weeks were used for categorization and for studies where iron exposure timing was not clear, a separate category “other” was created.
Type of neurodevelopmental outcome
Four categories of outcomes were used as follows: Cognition, motor development, behavior, and academic achievement. Studies that assessed outcomes of language, intelligence, memory, or mental development were categorized under cognitive outcomes and studies that assessed offspring temperament or socio-emotional development were categorized under behavioral outcomes.
Offspring age at assessment of neurodevelopmental outcome
We used five age group categories: Infancy (≤ 1 year), toddlers (> 1–5 years), childhood (> 5–11 years), adolescence (> 11–17 years) and adulthood (≥ 18 years).
Results
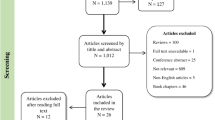
Of the 108 articles retained for full-text assessment, eligibility criteria for study inclusion/exclusion were applied and 81 articles were excluded leaving 19 observational studies and 8 RCTs for review, Fig. 1.
PRISMA flow chart of complete search strategy, literature identification, and study selection. Foot note: From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7): e1000097. https://doi.org/10.1371/journal.pmed1000097
Findings from studies
Study characteristics
Of the observational studies, 17 out of 19 were cohort studies, 1/19 was a case-control study [28] and 1/19 was of a correlational design [29]. They were published between 1986 and 2017 and included between 22 to 19,117 pregnant-women-and-child pairs.
RCTs were published between 2006 and 2017 and included 264 to 1166 intervention and control pairs. All experimental studies were RCTs; two were extensions of the same RCT [30, 31], three were extensions of another RCT with different neurodevelopmental outcome measures and ages of assessment [32,33,34] and one also took an observational approach into the RCT [33]. The majority of RCTs compared iron and folic acid supplementation to folic acid alone [32,33,34,35,36] while one RCT only, compared different doses of supplemented iron [37].
A summary of the characteristics and results of the studies are presented in Tables 2 and 3 and a visual representation of all study findings is shown in Figs. 2–4 and Supplemental files 5 and 6.
Observational studies on iron status in pregnancy and offspring cognitive outcomes. Findings from observational studies on the associations between iron status in pregnancy and offspring cognition presented in the graph according to the domains: (i) Type of iron status indicator for exposure measurement (top of the x-axis) (ii) exposure timing (bottom of the x-axis), and (iii) offspring age at neurodevelopmental assessment. The adjacent table presents information on each study on author and year (study ID), specific neurodevelopmental outcomes assessed, study sample size (Size) and quality score (QA). Studies are categorized according to offspring age at neurodevelopmental assessment: Infancy (≤ 1 year), toddlers (> 1–5 years), childhood (> 5–11 years), and further categorized according to study quality: High, medium and low quality. Bolded text: Specific items of cognition reported to be significantly associated. + Significant direct association. – Significant inverse association. ( + ) Association in direction of a direct association that did not reach significance. (−) Association in direction of an inverse association that did not reach significance. NS Non-significant associations and where effect estimates were not presented. Footnotes: 1 Exposure was measured persistently (at both time points of assessment). 2 Both Hb and Ht indicators were measured. 3 Hb levels measured as a mean value throughout pregnancy. 4 High Hb values (170–240 g/L) associated with lower scores. 5 sTfR measure in the Malawi cohort was in the direction of an inverse association while ZPP measure was in the direction of a direct one. Hb hemoglobin; Ht hematocrit; IBC iron binding capacity; MCV mean corpuscular volume; QA quality assessment score; SF serum ferritin; SI serum iron; sTfR soluble transferrin receptor; Size study sample size; TS transferrin saturation; ZPP zinc protoporphyrin
Observational studies on iron status in pregnancy and offspring motor developmental outcomes. Findings from observational studies on the associations between iron status in pregnancy and offspring motor development presented in the graph according to the domains (i) Type of iron status indicator for exposure measurement (top of the x-axis) (ii) exposure timing (bottom of the x-axis), and (iii) offspring age at neurodevelopmental assessment. The adjacent table presents information on each study on author and year (study ID), specific neurodevelopmental outcomes assessed, study sample size (Size) and quality score (QA). Studies are categorized according to offspring age at neurodevelopmental assessment: Infancy (≤ 1 years) and toddlers (> 1–5 years), and further categorized according to study quality: High, medium, and low quality. Bolded text: Specific items of cognition reported to be significantly associated. +Significant direct association. – Significant inverse association. ( + ) Association in direction of a direct association that did not reach significance. (−) Association in direction of an inverse association that did not reach significance. NS Non-significant associations and where effect estimates were not presented. NA No association found. U Significant inverted U-shaped association (lower and higher Hb levels were associated with lower motor scores) Footnotes: 1 Binary exposure (ID) was associated in the direction of a direct association while a continuous exposure measure of SF was in the direction of an inverse association. 2 ZPP measure in Ghana cohort was in the direction of an inverse association while sTfR was in the direction of a direct association. 3 Both Hb and Ht indicators were measured. 4 Hb levels measured as a mean value throughout pregnancy. Finger tapping item of motor development was in the direction of an inverse association unlike the rest of the motor development items. Hb hemoglobin; Ht hematocrit; IBC iron binding capacity; MCV mean corpuscular volume; QA quality assessment score; SF serum ferritin; SI serum Iron; sTfR soluble transferrin receptor; Size study sample size; TS transferrin saturation; ZPP zinc protoporphyrin
Observational studies on iron status in pregnancy and offspring behavioral outcomes. Findings from observational studies on the associations between iron status in pregnancy and offspring behavior presented in the graph according to the domains (i) Type of iron status indicator for exposure measurement (top of the x-axis) (ii) exposure timing (bottom of the x-axis), and (iii) offspring age at neurodevelopmental assessment. The adjacent table presents information on each study on author and year (study ID), specific neurodevelopmental outcomes assessed, study sample size (Size) and quality score (QA). Studies are categorized according to offspring age at neurodevelopmental assessment: Infancy (≤ 1 year) and toddlers (> 1–5 years), and further categorized according to study quality: high, medium and low quality. Bolded text: Specific items of cognition reported to be significantly associated. +Significant direct association. – Significant inverse association. (+) Association in direction of a direct association that did not reach significance. (−) Association in direction of an inverse association that did not reach significance. NS Non-significant associations and where effect estimates were not presented. NA No association found Footnotes: 1 Direction of association varied per assessed behavioral item. 2 Both Hb and Ht indicators were measured. ANS autonomous nervous system; Hb hemoglobin; Ht hematocrit; IBC iron binding capacity; MCV mean corpuscular volume; QA quality assessment score; SF serum ferritin; SI serum Iron; Size study sample size; TS transferrin saturation
Indicators of iron status and offspring neurodevelopment
Most observational studies (7/16) found direct [28, 38,39,40,41,42,43], 2/16 inverse [39, 44] and 1/16 inverted U-shaped [45] associations between Hb and offspring neurodevelopment, 2/6 found direct associations with SF [41, 46], 1/3 with SF + Hb [47], 1/2 with SI [43] and 1/1 with TS [46], TS + SF [46], IBC [29] or TS + SF + MCV [48].
Exposure timing and offspring neurodevelopment
One of four observational studies found direct association when iron status was measured in the 1st trimester [46], 2/9 when measured in the 2nd trimester [41, 46], 7/15 in the 3rd trimester [29, 38, 39, 42, 43, 46, 48], 1/10 at delivery/birth [48] and 3/3 when measured throughout pregnancy or persistently in more than one trimester [28, 41, 47]. Inverted U-shaped associations were reported when different iron indicators were measured in the 2nd trimester in 1/9 studies [45] and in 1/15 in the 3rd trimester [45]. Inverse associations were found in 1/15 studies in the 3rd trimester [44] and in 2/10 studies at birth [39, 44].
Only 1/7 RCTs found neurodevelopmental benefits of initiating iron supplementation preconceptionally [35], 1/7 found benefits when iron and folic acid compared to folic acid alone in the 2nd trimester [33] while 2/7 found adverse effects of supplementation in the 2nd trimester (supplemental file 6) [30, 31].
Type of neurodevelopmental outcome
Cognitive outcomes
Cognitive outcomes were investigated in 12 observational studies and 6 RCTs. In total, 4/12 observational studies found direct associations with offspring cognition [28, 39, 47, 48] and one also reported a significant inverse association with intelligence when Hb levels were measured at birth [39].
One of six RCTs, which took an observational approach in the RCT, also found a significant direct association with offspring mental scores [33].
Motor development outcome
Two of six observational studies with motor developmental outcomes reported direct associations with motor development [38, 48], and 1/6 reported an inverted U-shaped association [45].
One of five RCTs found improvements in motor development with the intervention but concluded that these effects were minor or chance findings [35].
Behavioral outcomes
Five of seven observational studies found direct [29, 41,42,43, 46] and 1/7 inverse associations between iron status using different types of iron indicators and offspring behavioral outcomes [44]. Most studies used the Neonatal Behavior Assessment Scale and the specific behavioral outcomes found to be significantly associated with iron status varied between studies (e.g., the outcome orientation was significantly associated in one study only while hyperactivity was associated in a different study).
Furthermore, 2/3 studies, of the same RCT, showed worse parent-reported behavioral scores among offspring of the iron supplemented mothers (supplemental file 6) [30, 31].
Academic achievement outcome
One study assessed offspring academic achievement and found a direct association (supplemental file 5) [40].
Offspring age at assessment of neurodevelopmental outcome
Seven of 12 observational studies found direct associations when outcomes were measured in infancy [29, 38, 41,42,43, 46, 47], 1/12 found an inverse [44] and 1/12 an inverted U-shaped association [45]. One of four RCTs found beneficial effects in infants [33]. In toddlers, 2/5 observational studies [28, 48] and 2/4 RCTs found beneficial associations [33, 35] while 2/4 RCTs reported adverse effects [30, 31]. No consistent results were seen in childhood and only one observational study reported on associations in adolescence [40]. No studies investigated associations between iron status during pregnancy and neurodevelopmental outcomes in adults (≥ 18 years of age).
Quality assessment results
Results of the quality assessment are reported in Supplemental file 4 per item on the Downs & Black checklist for each study. Scores for observational studies ranged from 10–21 and for RCTs from 20–26. Highest scores were obtained on Reporting Quality, followed by Internal Validity. Lowest scores were obtained for External Validity and Power. The total quality score for each study is presented in Tables 2 and 3. Fifteen of the 19 observational studies had medium and high quality and 4/19 had low quality.
Discussion
The present review found some evidence that a low gestational iron exposure, especially indicated using Hb and possibly when measured late in pregnancy, was associated with specific elements of adverse offspring neurodevelopment, particularly in infancy. Results of observational studies varied across quality score and thus, the heterogeneous results are likely not due to methodological issues. In the RCTs, the highest quality scores were found for studies with both direct and inverse associations, while studies that found no effects were of low and some medium quality. Therefore, the lack of intervention effects in 4/8 RCTs may rather depend on methodological weaknesses [32, 34, 36, 37].
Iron status indicators
Study results varied according to the iron status indicator but also within indicator. According to the WHO, ID can be categorized into three severity stages where different indicators’ levels are altered in different stages [2, 49] and thus may measure different physiologic conditions. Few studies used more specific indicators for iron and the majority used Hb, thus there was little support that the observed associations were attributable to real ID and the possibility of making firm conclusions related to ID was limited. For instance, some studies found direct associations with neurodevelopment using Hb but not other iron indicators [38, 41, 43, 44] and in some anemia was multifactorial and not due to ID [42, 45, 50]. Thus, caution should be taken in concluding that offspring neurodevelopment is related to ID from studies using Hb as its sole indicator. We did not exclude studies that only measured anemia (Hb) because (a) Although it is a poor proxy for iron status, it is still an indicator and (b) Because studies that use this indicator, as well as previous reviews on the topic, infer their results onto ID, and by including all these studies, we demonstrate here that one reason for the heterogeneity of results is due to not differentiating between more specific iron indicators and Hb, thus providing a clear direction for future research.
Timing of ID exposure
Results were generally not dependent on ID exposure timing during pregnancy. However, a greater number of studies found direct associations when iron status assessed during 3rd trimester [38, 40, 42], than earlier in pregnancy, suggesting that late gestation iron status may be of particular importance for offspring neurodevelopment, in agreement with findings from other studies that iron uptake and myelination are increased during this period [51]. Animal studies have suggested that ID—also early in pregnancy—influences myelination in the developing brain [52], thus the results observed for 3rd trimester ID measures in these human studies could have resulted from ID starting early and continuing through pregnancy, and could explain why our results did not seem dependent on exposure timing. In agreement, one study found persistent ID anemia (IDA), but not IDA at one pregnancy point, to be associated with cognition [47]. Moreover, there could be several periods during brain genesis of different sensitivity to iron in different brain regions accounting for the heterogeneous findings [12, 51, 53,54,55]. Consistantly, two studies found direct associations between ID—using different iron indicators—in different pregnancy trimesters and offspring outcomes [46, 48]. Finally, exposure timing could be highly dependent on the iron status indicator used in each of the studies. The great variety in these indicators might have thus limited observation of a trend in the timing of ID exposure, if one was present. This can be seen from the study by Hernandez-Martinez et al. (2011) [46] who showed that ID was associated with different behavioral items at different pregnancy stages as well as when using different indicators of iron status. Thus, we show that exploration of domains of exposure timing, iron status indicators, and neurodevelopmental outcomes is needed and understanding the relationship between pregnancy iron status or anemia and offspring neurodevelopment should always take these into consideration.
Neurodevelopmental outcomes
Associations between ID and neurodevelopment varied depending on the specific neurodevelopmental outcome, and direct associations were observed particularly for behavior and cognition, and less evidently for other outcomes. However, associations with cognition were few and mainly seen in studies assessing Hb levels. Results were also few for motor development, and in the absence of more studies, there is overall little evidence for an association. One study only, assessed offspring academic achievement, and despite its high methodological quality, this study assessed ID using Hb levels, and thus the results should—as discussed earlier—be regarded with caution, and need to be confirmed using other ID indicators [40]. Several studies found direct associations between ID indicators and different behavioral outcomes but results varied across trimesters and with the type of iron status indicator. Moreover, 2 RCTs reported inverse associations, thus neither the direction of the associations nor the specific behavioral outcomes affected were clear from the included studies [30, 31] and heterogeneous findings may also relate to challenges in assessing behavior in early life i.e., infancy [56]. Finally, maternal iron overload may potentially also adversely affect offspring health, as reported in both human and animal studies [57]. Indeed, a few of the observational studies found high Hb to be associated with adverse motor development and behavior [44, 45], and future studies should examine this further, especially because current clinical practices follow supplementation in pregnancy with iron assuming favorable health outcomes, only [24, 58].
Offspring age at assessment of neurodevelopmental outcome
Findings from the included studies suggest that associations may persist beyond infancy into childhood, and potentially further into adolescence. However, the scarcity of human studies following offspring past infancy and childhood poses a serious limitation to our current knowledge regarding long term neurodevelopmental effects of pregnancy ID. Because neurodevelopmental skills emerge at different rates in early life [53, 59, 60], the lack of a relation between iron status and cognition in infancy does not exclude the possibility of delays at school age [59], as suggested by Fararouei et al. [40].
Strengths and limitations of this review
This systematic literature review has several strengths: (a) We followed PRISMA guidelines and used recognized tools for data extraction and quality assessment; (b) All study findings were visually presented to the reader which we believe has minimized reviewer biases as much as possible. We also report the following limitations: a) We cannot exclude the possibility of missing some studies in our search. However, we searched several databases, hand-searched reference lists and searched grey literature. We included both observational studies and RCTs with no restrictions to publication dates and included studies from both developing (16 studies) and developed economy countries (11 studies) [61]. We therefore believe that our search most likely was complete. We identified one study where the English abstract suggested relevant findings, but the study was only available in Chinese and thus was not included in our review [62]; (b) We could not conduct a meta-analysis due to the heterogeneous nature of the studies; (c) We could expect studies that used the same cohorts or RCTs to be subject to publication or confirmation biases for reaching consistent results. However, their quality scores varied and they had different hypotheses, thus we do not expect that our conclusions were biased by this. However, we note that this is an important consideration when the reader looks at the number of studies reviewed. We point out that the reason we did not only include one of the several studies which were based on the same cohort or trial, is due to the different exposures and outcomes investigated which puts these studies in different categories under our four selected domains. And since we aimed at investigating the specific domains, we chose to keep all studies stemming from the same cohort or trial; (d) Although evidence from literature shows that micronutrient deficiencies are more common and possibly more severe in developing economy countries—which can in turn influence the effects observed on offspring neurodevelopment—we have not compared studies on the severity of ID or anemia, because the definitions of the two varied greatly across studies. We however notice that significant associations were observed in both developed and developing economy settings in the studies included in our review, with no clear patterns depending on the setting; (e) Small sample sizes and methodological issues in some of the included studies could have limited analyses of reaching significance, and in some studies with small sample sizes and high rates of loss-to-follow-up, selection bias could be an issue which is a threat to the validity and generalizability of their findings. We however account for biases and errors, as well as power calculation and sample size in our quality assessment, and thus these issues are reflected in the quality score of each study. Finally, (f) Of the RCTs, two used a lower iron dose than the remaining, however we did not see a pattern of outcomes based on the dose, where both studies showed significant associations in some domains and not others, and where the remaining studies also showed similar findings. Thus, we do not see this affecting the findings of the review.
Agreement with previous reviews
The current review reached conclusions that were both consistent and contrasting with conclusions from the two previously published reviews in this area [23, 24]. Veena et al. concluded that there is a little support for or lack of effect of iron on children’s cognitive funtion [24]; in contrast, we conclude that pregnancy iron status may be associated with certain, but not all offspring neurodevelopmental outcomes. Thus, consistently with the conclusion drawn by Iglesias et al. [23], we argue that there seems to be some evidence that pregnancy iron status may be associated with offspring behavior, cognition, and academic achievement but in contrast, we found no evidence on motor development. We also note that there is large heterogeneity in the results and a need for more studies, particularly well-performed RCTs. We attribute our contrasting evidence to our focus on the four domains and to the set eligibility criteria. Studies where exposures were self-reported or the effects of iron alone could not be assessed were included in previous reviews but were excluded in ours based on the eligibility criteria. We believe that this was reasonable and did not bias our conclusions.
Conclusions and further challenges
The present review of observational studies and RCTs on prenatal iron exposure and later neurodevelopment suggest that there is some evidence that low pregnancy iron, particularly in the 3rd trimester, may adversely influence offspring neurodevelopment. However, as most previous research used Hb as an indicator for iron status, inferring results to ID should be done with caution. We thus also demonstrate the weakness of available literature to date in relation to use of more specific iron status indicators than just Hb, and we believe this is an important conclusion especially because previous reviews and studies do not clearly differentiate between iron status and anemia, which we show has led to the inconclusiveness of evidence on the topic, to date. Results were few and heterogeneous regarding associations beyond the age of early childhood and there is a need for more studies that (a) Perform multiple iron status measurements during all stages of pregnancy, use different indicators of iron status and look at different neurodevelopmental outcomes separately from one another; (b) Follow offspring into adolescence and adulthood ages to confirm potential changes in associations over time and specifically with the offspring academic achievement; (c) Investigate associations of iron overload in pregnancy on offspring neurodevelopment and (d) Future high quality observational studies and RCTs should assess offspring behavior, especially those that assess the same behavioral outcomes found to be significantly associated in our review.
References
World Health Organization. WHO | Micronutrient deficiencies [http://www.who.int/nutrition/topics/ida/en/]. World Health Organization, 2015.
United Nations Children’s Fund. Iron Deficiency Anaemia Assessment, Prevention, and Control A guide for programme managers. 2001;1–114.
World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. WHO Rep. 2016;1–120.
World Health Organization. The Global Prevalence of Anaemia in 2011. WHO Rep. 2011;1–44.
World Health Organization. Evaluating the public health significance of micronutrient malnutrition. In. Allen L, de Benoist B, Dary O, Hurrell R, editors. Guidelines on food fortification with micronutrients. Geneva: Switzerland; 2006;41–92.
Connor JR, Menzies SL. Altered cellular distribution of iron in the central nervous system of myelin deficient rats. Neuroscience. 1990;34:265–71.
Kwik-Uribe CL, Gietzen D, German JB, Golub MS, Keen CL. Chronic marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. J Nutr. 2000;130:2821–30.
Nelson C, Erikson K, Piñero DJ, Beard JL. In vivo dopamine metabolism is altered in iron-deficient anemic rats. J Nutr. 1997;127:2282–8.
Erikson KM, Shihabi ZK, Aschner JL, Aschner M. Manganese accumulates in iron-deficient rat brain regions in a heterogeneous fashion and is associated with neurochemical alterations. Biol Trace Elem Res. 2002;87:143–56.
Beard JL, Connor JR. Iron status and neural functioning. Annu Rev Nutr. 2003;23:41–58.
Tran PV, Fretham SJB, Carlson ES, Georgieff MK. Long-term reduction of hippocampal brain-derived neurotrophic factor activity after fetal-neonatal iron deficiency in adult rats. Pediatr Res. 2009;65:493–8.
Georgieff MK. Iron in the brain. Neoreviews. 2006;7:e344–52.
Ibi M, Sawada H, Nakanishi M, Kume T, Katsuki H, Kaneko S, et al. Protective effects of 1α,25-(OH)2D3 against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. 2001;40:761–71.
Srivastava DPP, Waters EMM, Mermelstein PGG, Kram r EAA, Shors TJJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. J Neurosci. 2011;31:16056–63.
Li D. Effects of iron deficiency on iron distribution and gamma-aminobutyric acid (GABA) metabolism in young rat brain tissues. Hokkaido Igaku Zasshi. 1998;73:215–25.
Taneja V, Mishra K, Agarwal KN. Effect of early iron deficiency in rat on the gamma-aminobutyric acid shunt in brain. J Neurochem. 1986;46:1670–4.
Connor JR, Menzies SL, Burdo JRBP. Iron and iron management proteins in neurobiology. Pediatr Neurol. 2001;25:118–29.
Beard JL, Connor JR, Jones BC. Iron in the brain. Nutr Rev. 1993;51:157–70.
Niillard SA. Ribonucleotide reductase in developing brain. J Biol Chem. 1972;247:2395–400.
Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–65.
Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev. 2010;20:327–48.
Honda K, Casadesus G, Petersen RB, Perry G, Smith MA. Oxidative stress and redox-active iron in Alzheimer’s Disease. Ann N Y Acad Sci. 2004;1012:179–82.
Iglesias L, Canals J, Arija V. Effects of prenatal iron status on child neurodevelopment and behavior: a systematic review. Crit Rev Food Sci Nutr. 2018;58:1604–14.
Veena SR, Gale CR, Krishnaveni GV, Kehoe SH, Srinivasan K, Fall CH. Association between maternal nutritional status in pregnancy and offspring cognitive function during childhood and adolescence; a systematic review. BMC Pregnancy Childbirth. 2016;16:220.
Higgins JPT GSS. Cochrane Handbook for Systematic Reviews of Interventions | Cochrane Training [http://training.cochrane.org/handbook]. The Cochrane Collaboration. 2013.
Downs SHH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Heal. 1998;52:377–84.
NIH - National Library of Medicine. Pregnancy Trimesters - National Library of Medicine - PubMed Health [https://www.ncbi.nlm.nih.gov/pubmedhealth/PMHT0023078/].
Ellman LM, Vinogradov S, Kremen WS, Poole JH, Kern DM, Deicken RF, et al. Low maternal hemoglobin during pregnancy and diminished neuromotor and neurocognitive performance in offspring with schizophrenia. Schizophr Res. 2012;138:81–7.
Vaughn J, Brown J, Carter JP. The effects of maternal anemia on infant behavior. J Natl Med Assoc. 1986;78:963–8.
Zhou SJ, Gibson RA, Crowther CA, Baghurst P, Makrides M. Effect of iron supplementation during pregnancy on the intelligence quotient and behavior of children at 4 y of age: Long-term follow-up of a randomized controlled trial. Am J Clin Nutr. 2006;83:1112–7.
Parsons A, Zhou S, Spurrier N, Makrides M. Effect of iron supplementation during pregnancy on the behaviour of children at early school age: long-term follow-up of a randomised controlled trial. Br J Nutr. 2008;99:1133–9.
Li Q, Yan H, Zeng L, Cheng Y, Liang W, Dang S, et al. Effects of maternal multimicronutrient supplementation on the mental development of infants in rural western China: Follow-up evaluation of a double-blind, randomized, controlled trial. Pediatrics. 2009;123:e685–92.
Chang S, Zeng L, Brouwer ID, Kok FJ. Effect of iron deficiency anemia in pregnancy on child mental development in rural China. Pediatrics. 2013;131:e755–63.
Li C, Zeng L, Wang D, Yang W, Dang S, Zhou J, et al. Prenatal micronutrient supplementation is not associated with intellectual development of young school-aged children. J Nutr. 2015;145:1844–9.
Nguyen PH, Gonzalez-Casanova I, Young MF, Truong TV, Hoang H, Nguyen H, et al. Preconception micronutrient supplementation with iron and folic acid compared with folic acid alone affects linear growth and fine motor development at 2 years of age: a randomized controlled trial in Vietnam. J Nutr. 2017;147:1593–601.
Angulo-Barroso R, Li M, Santos D, Bian Y, Sturza J, Jiang Y, et al. Iron supplementation in pregnancy or infancy and motor development: a randomized controlled trial. Pediatrics. 2016;137: e20153547.
Tofail F, Persson L, Arifeen S, Hamadani J, Mehrin F, Ridout D, et al. Effects of prenatal food and micronutrient supplementation on infant development: a randomized trial from the maternal and infant nutrition interventions, Matlab (MIMIMat) study. Am J Clin Nutr. 2008;87:704–11.
Tran TD, Tran T, Simpson JA, Tran HT, Nguyen TT, Hanieh S, et al. Infant motor development in rural Vietnam and intrauterine exposures to anaemia, iron deficiency and common mental disorders: a prospective community-based study. BMC Pregnancy Childbirth. 2014;14:8.
Naeye RL, Peters EC. Antenatal hypoxia and low IQ values. Am J Dis Child. 1987;141:50–4.
Fararouei M, Robertson C, Whittaker J, Sovio U, Ruokonen A, Pouta A, et al. Maternal Hb during pregnancy and offspring’s educational achievement: a prospective cohort study over 30 years. Br J Nutr. 2010;104:1363–8.
Oyemade UJ, Cole OJ, Johnson AA, Knight EM, Westney OE, Laryea H, et al. Prenatal predictors of performance on the Brazelton Neonatal Behavioral Assessment Scale. J Nutr. 1994;124(6 Suppl):1000S–1005S.
Menon KC, Ferguson EL, Thomson CD, Gray AR, Zodpey S, Saraf A, et al. Effects of anemia at different stages of gestation on infant outcomes. Nutrition. 2016;32:61–5.
Wachs TD, Pollitt E, Cueto S, Jacoby E, Creed-Kanashiro H. Relation of neonatal iron status to individual variability in neonatal temperament. Dev Psychobiol. 2005;46:141–53.
Aranda N, Hernández-Martínez C, Arija V, Ribot B, Canals J. Haemoconcentration risk at the end of pregnancy: effects on neonatal behaviour. Public Health Nutr. 2017;20:1405–13.
Mireku M, Davidson L, Koura G, Ouédraogo S, Boivin M, Xiong X, et al. Prenatal hemoglobin levels and early cognitive and motor functions of one-year-old children. Pediatrics. 2015;136:e76–83.
Hernández-Martínez C, Canals J, Aranda N, Ribot B, Escribano J, Arija V. Effects of iron deficiency on neonatal behavior at different stages of pregnancy. Early Hum Dev. 2011;87:165–9.
Tran TD, Biggs B-A, Tran TD, Simpson JA, Hanieh S, Dwyer T. Impact on infants’ cognitive development of antenatal exposure to iron deficiency disorder and common mental disorders. PLoS ONE. 2013;8:e74876.
Berglund SK, Torres-Espinola FJ, Garcia-Valdes L, Segura MT, Martinez-Zaldivar C, Padilla C. et al. The impacts of maternal iron deficiency and being overweight during pregnancy on neurodevelopment of the offspring. Br J Nutr. 2017;118:533–40.
Lynch S. The rationale for selecting and standardizing iron status indicators. World Health Organization. 2012.
Mireku MO, Davidson LL, Boivin MJ, Zoumenou R, Massougbodji A, Cot M, et al. Prenatal iron deficiency, neonatal ferritin, and infant cognitive function. Pediatrics. 2016;138:e20161319.
Wachs TD, Georgieff M, Cusick S, McEwen BS. Issues in the timing of integrated early interventions: contributions from nutrition, neuroscience, and psychological research. Ann N Y Acad Sci. 2014;1308:89–106.
Morath DJ, Mayer-Pröschel M. Iron deficiency during embryogenesis and consequences for oligodendrocyte generation in vivo. Dev Neurosci. 2002;24:197–207.
Doom JR, Georgieff MK. Striking while the iron is hot: Understanding the biological and neurodevelopmental effects of iron deficiency to optimize intervention in early childhood. Curr Pediatr Rep. 2014;2:291–8.
Beard L, Kretchmer N, Beard JL, Carlson S. The role of nutrition in the development of normal cognition. Am J Clin Nutr. 1996;63:997S–1001S.
Bailey L, West KP, Black RP. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab. 2015;66:22–33.
Jones SM, Darling-Churchill KE, Halle TG. Assessing early childhood social and emotional development: Key conceptual and measurement issues. J Appl Dev Psychol. 2016;45:42–8.
Brown VJ. Reproductive toxicity: too much of a good thing? Environ Health Perspect. 2006;114:A578.
Taylor R, Fealy S, Bisquera A, Smith R, Collins C, Evans T-J, et al. Effects of nutritional interventions during pregnancy on infant and child cognitive outcomes: a systematic review and meta-analysis. Nutrients. 2017;9:1265.
Fernald LC, Kariger P, Engle P, Raikes A. Examining early child development in low-income countries: a toolkit for the assessment of children in the first five years of life. The World Bank, 2009;1–133.
Pollitt E, Triana N. Stability, Predictive Validity, and Sensitivity of Mental and Motor Development Scales and Pre-School Cognitive Tests among Low-Income Children in Developing Countries. Food Nutr Bull. 1999;20:45–52.
Dji. Country Classification System. Dow Jones Indexes. 2011;1–3.
Yang L, Ren AG, Liu JM, Ye RW, Hong SX. Influence of hemoglobin level during early gestation on the development of cognition of pre-school children. Zhonghua Liu Xing Bing Xue Za Zhi. 2010;31:1353–8.
Wasserman GA, Graziano JH, Factor-Litvak P, Popovac D, Morina N, Musabegovic A, et al. Consequences of lead exposure and iron supplementation on childhood development at age 4 years. Neurotoxicol Teratol. 1994;16:233–40.
Wasserman G, Graziano JH, Factor-Litvak P, Popovac D, Morina N, Musabegovic A, et al. Independent effects of lead exposure and iron deficiency anemia on developmental outcome at age 2 years. J Pediatr. 1992;121(5 Pt 1):695–703.
Rioux FM, Bélanger-Plourde J, Leblanc PL, Vigneau F. Relationship Between Maternal DHA and Iron Status And Infants' Cognitive Performance. Canadian Journal of Dietetic Practice and Research. 2011;72:e140–e146.
Lewis SJ, Bonilla C, Brion M-J, Lawlor DA, Gunnell D, Ben-Shlomo Y. et al. Maternal iron levels early in pregnancy are not associated with offspring IQ score at age 8, findings from a Mendelian randomization study. European Journal of Clinical Nutrition. 2014;68:496–502.
Prado EL, Abbeddou S, Adu-Afarwuah S, Arimond M, Ashorn M, Ashorn U. et al. Predictors and pathways of language and motor development in four prospective cohorts of young children in Ghana, Malawi, and Burkina Faso. Journal of Child Psychology and Psychiatry. 2017;58:1264–1275.
Acknowledgements
We acknowledge the contribution of the statistician Volkert Siersma from the University of Copenhagen to our work through providing statistical help and Gabriel Gulis from the University of Southern Denmark for supervising the writing process.
Funding
Twelve months of under-graduate research stipend was received from the Lundbeck Foundation.
Author information
Authors and Affiliations
Contributions
JJ, IOS, and BLH were responsible for project conception and developed the overall research plan. JJ and MS conducted the search. JJ analyzed data. JJ, IOS, and BLH wrote the paper. JJ had primary responsibility for final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Janbek, J., Sarki, M., Specht, I.O. et al. A systematic literature review of the relation between iron status/anemia in pregnancy and offspring neurodevelopment. Eur J Clin Nutr 73, 1561–1578 (2019). https://doi.org/10.1038/s41430-019-0400-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-019-0400-6
This article is cited by
-
Sex-specific association between maternal mild anemia and children’s behavioral development: a birth cohort study
European Child & Adolescent Psychiatry (2024)
-
RAPIDIRON Trial follow-up study — the RAPIDIRON-KIDS Study: protocol of a prospective observational follow-up study
Trials (2023)
-
Maternal iron status in early pregnancy and DNA methylation in offspring: an epigenome-wide meta-analysis
Clinical Epigenetics (2022)
-
Anaemia and iron deficiency in pregnancy and adverse perinatal outcomes in Southern India
European Journal of Clinical Nutrition (2020)
-
Safety of intravenous iron isomaltoside for iron deficiency and iron deficiency anemia in pregnancy
Archives of Gynecology and Obstetrics (2020)