Abstract
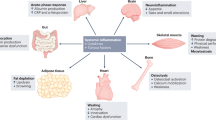
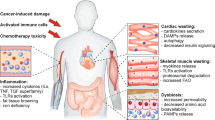
Cancer cachexia is a multifactorial syndrome that is characterised by a loss of skeletal muscle mass, is commonly associated with adipose tissue wasting and malaise, and responds poorly to therapeutic interventions. Although cachexia can affect patients who are severely ill with various malignant or non-malignant conditions, it is particularly common among patients with pancreatic cancer. Pancreatic cancer often leads to the development of cachexia through a combination of distinct factors, which, together, explain its high prevalence and clinical importance in this disease: systemic factors, including metabolic changes and pathogenic signals related to the tumour biology of pancreatic adenocarcinoma; factors resulting from the disruption of the digestive and endocrine functions of the pancreas; and factors related to the close anatomical and functional connection of the pancreas with the gut. In this review, we conceptualise the various insights into the mechanisms underlying pancreatic cancer cachexia according to these three dimensions to expose its particular complexity and the challenges that face clinicians in trying to devise therapeutic interventions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Fearon, K., Strasser, F., Anker, S. D., Bosaeus, I., Bruera, E., Fainsinger, R. L. et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 12, 489–495 (2011).
Baracos, V. E., Martin, L., Korc, M., Guttridge, D. C. & Fearon, K. C. H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 4, 17105 (2018).
Martin, L., Birdsell, L., MacDonald, N., Reiman, T., Clandinin, M. T., McCargar, L. J. et al. Cancer cachexia in the age of obesity: skeletal muscle depletion Is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 31, 1539–1547 (2013).
Minicozzi, P., Cassetti, T., Vener, C. & Sant, M. Analysis of incidence, mortality and survival for pancreatic and biliary tract cancers across Europe, with assessment of influence of revised European age standardisation on estimates. Cancer Epidemiol. 55, 52–60 (2018).
Hendifar, A. E., Chang, J. I., Huang, B. Z., Tuli, R. & Wu, B. U. Cachexia, and not obesity, prior to pancreatic cancer diagnosis worsens survival and is negated by chemotherapy. J. Gastrointest. Oncol. 9, 17–23 (2018).
Mitsunaga S., Kasamatsu E. & Machii K. Incidence and frequency of cancer cachexia during chemotherapy for advanced pancreatic ductal adenocarcinoma. Support Care Cancer 28, 5271–5279 (2020)
Kays, J. K., Shahda, S., Stanley, M., Bell, T. M., O’Neill, B. H., Kohli, M. D. et al. Three cachexia phenotypes and the impact of fat-only loss on survival in FOLFIRINOX therapy for pancreatic cancer. J. Cachexia Sarcopenia Muscle 9, 673–684 (2018).
Choi, Y., Oh, D. Y., Kim, T. Y., Lee, K. H., Han, S. W., Im, S. A. et al. Skeletal muscle depletion predicts the prognosis of patients with advanced pancreatic cancer undergoing palliative chemotherapy, independent of body mass index. PLoS ONE 10, e0139749 (2015).
Bachmann, J., Heiligensetzer, M., Krakowski-Roosen, H., Buchler, M. W., Friess, H. & Martignoni, M. E. Cachexia worsens prognosis in patients with resectable pancreatic cancer. J. Gastrointest. Surg. 12, 1193–1201 (2008).
Bauer, M. R., Bright, E. E., MacDonald, J. J., Cleary, E. H., Hines, O. J. & Stanton, A. L. Quality of life in patients with pancreatic cancer and their caregivers. Pancreas 47, 368–375 (2018).
Hagensen, A., London, A. E., Phillips, J. J., Helton, W. S., Picozzi, V. J. & Blackmore, C. C. Using experience-based design to improve the care experience for patients with pancreatic cancer. J. Oncol. Pract. 12, e1035–e1041 (2016).
Waddell, N., Pajic, M., Patch, A. M., Chang, D. K., Kassahn, K. S., Bailey, P. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501 (2015).
Ying, H., Kimmelman, A. C., Lyssiotis, C. A., Hua, S., Chu, G. C., Fletcher-Sananikone, E. et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 149, 656–670 (2012).
Guillaumond, F., Leca, J., Olivares, O., Lavaut, M.-N., Vidal, N., Berthezène, P. et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc. Natl Acad. Sci. USA 110, 3919–3924 (2013).
Chen, Y., Cairns, R., Papandreou, I., Koong, A. & Denko, N. C. Oxygen consumption can regulate the growth of tumors, a new perspective on the Warburg effect. PLoS ONE 4, e7033 (2009).
Wang, F., Liu, H., Hu, L., Liu, Y., Duan, Y., Cui, R. et al. The Warburg effect in human pancreatic cancer cells triggers cachexia in athymic mice carrying the cancer cells. BMC Cancer 18, 360 (2018).
Son, J., Lyssiotis, C. A., Ying, H., Wang, X., Hua, S., Ligorio, M. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013).
Roux, C., Riganti, C., Borgogno, S. F., Curto, R., Curcio, C., Catanzaro, V. et al. Endogenous glutamine decrease is associated with pancreatic cancer progression. Oncotarget 8, 95361–95376 (2017).
Vasseur, S., Tomasini, R., Tournaire, R. & Iovanna, J. L. Hypoxia induced tumor metabolic switch contributes to pancreatic cancer aggressiveness. Cancers (Basel) 2, 2138–2152 (2010).
Sousa, C. M., Biancur, D. E., Wang, X., Halbrook, C. J., Sherman, M. H., Zhang, L. et al. Pancreatic stellate cells support tumour metabolism through autophagic alanine secretion. Nature 536, 479–483 (2016).
Mayers, J. R., Wu, C., Clish, C. B., Kraft, P., Torrence, M. E., Fiske, B. P. et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nat. Med. 20, 1193–1198 (2014).
Neinast, M. D., Jang, C., Hui, S., Murashige, D. S., Chu, Q., Morscher, R. J. et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metabolism 29, 417–429.e414 (2019).
Mayers, J. R., Torrence, M. E., Danai, L. V., Papagiannakopoulos, T., Davidson, S. M., Bauer, M. R. et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 353, 1161–1165 (2016).
Lee, J. H., Cho, Y. R., Kim, J. H., Kim, J., Nam, H. Y., Kim, S. W. et al. Branched-chain amino acids sustain pancreatic cancer growth by regulating lipid metabolism. Exp. Mol. Med. 51, 1–11 (2019).
Das, S. K., Eder, S., Schauer, S., Diwoky, C., Temmel, H., Guertl, B. et al. Adipose triglyceride lipase contributes to cancer-associated cachexia. Science 333, 233–238 (2011).
Ryden, M., Agustsson, T., Laurencikiene, J., Britton, T., Sjolin, E., Isaksson, B. et al. Lipolysis–not inflammation, cell death, or lipogenesis–is involved in adipose tissue loss in cancer cachexia. Cancer 113, 1695–1704 (2008).
Shaw, J. H. & Wolfe, R. R. Fatty acid and glycerol kinetics in septic patients and in patients with gastrointestinal cancer. The response to glucose infusion and parenteral feeding. Ann. Surg. 205, 368–376 (1987).
Wang, F., Kumagai-Braesch, M., Herrington, M. K., Larsson, J. & Permert, J. Increased lipid metabolism and cell turnover of MiaPaCa2 cells induced by high-fat diet in an orthotopic system. Metabolism 58, 1131–1136 (2009).
Agustsson, T., Ryden, M., Hoffstedt, J., van Harmelen, V., Dicker, A., Laurencikiene, J. et al. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 67, 5531–5537 (2007).
Russell, S. T. & Tisdale, M. J. Effect of a tumour-derived lipid-mobilising factor on glucose and lipid metabolism in vivo. Br. J. Cancer 87, 580–584 (2002).
Russell, S. T., Zimmerman, T. P., Domin, B. A. & Tisdale, M. J. Induction of lipolysis in vitro and loss of body fat in vivo by zinc-alpha2-glycoprotein. Biochim. Biophys. Acta 59–68, 2004 (1636).
Bao, Y., Bing, C., Hunter, L., Jenkins, J. R. & Wabitsch, M. Trayhurn P. Zinc-α2-glycoprotein, a lipid mobilizing factor, is expressed and secreted by human (SGBS) adipocytes. FEBS Lett. 579, 41–47 (2005).
Bing, C., Bao, Y., Jenkins, J., Sanders, P., Manieri, M., Cinti, S. et al. Zinc-α2-glycoprotein, a lipid mobilizing factor, is expressed in adipocytes and is up-regulated in mice with cancer cachexia. Proc. Natl Acad. Sci. USA 101, 2500–2505 (2004).
Kulyte, A., Lorente-Cebrian, S., Gao, H., Mejhert, N., Agustsson, T., Arner, P. et al. MicroRNA profiling links miR-378 to enhanced adipocyte lipolysis in human cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 306, E267–E274 (2014).
Sagar, G., Sah, R. P., Javeed, N., Dutta, S. K., Smyrk, T. C., Lau, J. S. et al. Pathogenesis of pancreatic cancer exosome-induced lipolysis in adipose tissue. Gut 65, 1165–1174 (2016).
Rohm M., Zeigerer A., Machado J. & Herzig S. Energy metabolism in cachexia. EMBO Rep. 20, e47258 (2019)
Mitsunaga, S., Ikeda, M., Shimizu, S., Ohno, I., Furuse, J., Inagaki, M. et al. Serum levels of IL-6 and IL-1beta can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br. J. Cancer 108, 2063–2069 (2013).
Petruzzelli, M., Schweiger, M., Schreiber, R., Campos-Olivas, R., Tsoli, M., Allen, J. et al. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 20, 433–447 (2014).
Sah, R. P., Sharma, A., Nagpal, S., Patlolla, S. H., Sharma, A., Kandlakunta, H. et al. Phases of metabolic and soft tissue changes in months preceding a diagnosis of pancreatic ductal adenocarcinoma. Gastroenterology 156, 1742–1752 (2019).
Bing, C., Russell, S. T., Beckett, E. E., Collins, P., Taylor, S., Barraclough, R. et al. Expression of uncoupling proteins-1, -2 and -3 mRNA is induced by an adenocarcinoma-derived lipid-mobilizing factor. Br. J. Cancer 86, 612–618 (2002).
Falconer, J. S., Fearon, K. C., Plester, C. E., Ross, J. A. & Carter, D. C. Cytokines the acute-phase response, and resting energy expenditure in cachectic patients with pancreatic cancer. Ann. Surg. 219, 325–331 (1994).
Gonzalez-Bulnes, A., Fujiwara, Y., Kobayashi, T., Chayahara, N., Imamura, Y., Toyoda, M. et al. Metabolomics evaluation of serum markers for cachexia and their intra-day variation in patients with advanced pancreatic cancer. PLoS ONE 9, e113259 (2014).
Bye, A., Wesseltoft-Rao, N., Iversen, P. O., Skjegstad, G., Holven, K. B., Ulven, S. et al. Alterations in inflammatory biomarkers and energy intake in cancer cachexia: a prospective study in patients with inoperable pancreatic cancer. Med. Oncol. 33, 54 (2016).
Bachmann, J., Buchler, M. W., Friess, H. & Martignoni, M. E. Cachexia in patients with chronic pancreatitis and pancreatic cancer: impact on survival and outcome. Nutr. Cancer 65, 827–833 (2013).
Talar-Wojnarowska, R., Gasiorowska, A., Smolarz, B., Romanowicz-Makowska, H., Kulig, A. & Malecka-Panas, E. Clinical significance of interleukin-6 (Il-6) gene polymorphism and Il-6 serum level in pancreatic adenocarcinoma and chronic pancreatitis. Dig. Dis. Sci. 54, 683–689 (2008).
Miura, T., Mitsunaga, S., Ikeda, M., Shimizu, S., Ohno, I., Takahashi, H. et al. Characterization of patients with advanced pancreatic cancer and high serum interleukin-6 levels. Pancreas 44, 756–763 (2015).
Talbert, E. E., Lewis, H. L., Farren, M. R., Ramsey, M. L., Chakedis, J. M., Rajasekera, P. et al. Circulating monocyte chemoattractant protein-1 (MCP-1) is associated with cachexia in treatment-naive pancreatic cancer patients. J. Cachexia Sarcopenia Muscle 9, 358–368 (2018).
Hou, Y.-C., Wang, C.-J., Chao, Y.-J., Chen, H.-Y., Wang, H.-C., Tung, H.-L. et al. Elevated serum interleukin-8 level correlates with cancer-related cachexia and sarcopenia: An indicator for pancreatic cancer outcomes. J. Clin. Med. 7, 502 (2018).
Martignoni, M. E. Role of mononuclear cells and inflammatory cytokines in pancreatic cancer-related cachexia. Clin. Cancer Res. 11, 5802–5808 (2005).
Zhang, D., Zhou, Y., Wu, L., Wang, S., Zheng, H., Yu, B. et al. Association of IL-6 gene polymorphisms with cachexia susceptibility and survival time of patients with pancreatic cancer. Ann. Clin. Lab. Sci. 38, 113–119 (2008).
Egberts, J. H., Cloosters, V., Noack, A., Schniewind, B., Thon, L., Klose, S. et al. Anti-tumor necrosis factor therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 68, 1443–1450 (2008).
de Matos-Neto, E. M., Lima, J. D. C. C., de Pereira, W. O., Figuerêdo, R. G., Riccardi, D. M. D. R., Radloff, K. et al. Systemic inflammation in cachexia - Is tumor cytokine expression profile the culprit? Front. Immunol. 6, 629–629 (2015).
Shimada, M., Andoh, A., Araki, Y., Fujiyama, Y. & Bamba, T. Ligation of the Fas antigen stimulates chemokine secretion in pancreatic cancer cell line PANC-11. J. Gastroenterol. Hepatol. 16, 1060–1067 (2001).
Delitto, D., Judge, S. M., Delitto, A. E., Nosacka, R. L., Rocha, F. G., DiVita, B. B. et al. Human pancreatic cancer xenografts recapitulate key aspects of cancer cachexia. Oncotarget 8, 1177–1189 (2017).
Gerber, M. H., Underwood, P. W., Judge, S. M., Delitto, D., Delitto, A. E., Nosacka, R. L. et al. Local and systemic cytokine profiling for pancreatic ductal adenocarcinoma to study cancer cachexia in an era of precision medicine. Int. J. Mol. Sci. 19, 3836 (2018).
Haugen, F., Labori, K. J., Noreng, H. J., Buanes, T., Iversen, P. O. & Drevon, C. A. Altered expression of genes in adipose tissues associated with reduced fat mass in patients with pancreatic cancer. Arch. Physiol. Biochem. 117, 78–87 (2011).
Bonetto, A., Aydogdu, T., Jin, X., Zhang, Z., Zhan, R., Puzis, L. et al. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 303, E410–E421 (2012).
Pettersen, K., Andersen, S., Degen, S., Tadini, V., Grosjean, J., Hatakeyama, S. et al. Cancer cachexia associates with a systemic autophagy-inducing activity mimicked by cancer cell-derived IL-6 trans-signaling. Sci. Rep. 7, 2046 (2017).
Ma, J. F., Sanchez, B. J., Hall, D. T., Tremblay, A. K., Di Marco, S. & Gallouzi, I. E. STAT3 promotes IFNgamma/TNFalpha-induced muscle wasting in an NF-kappaB-dependent and IL-6-independent manner. EMBO Mol. Med. 9, 622–637 (2017).
Hall, D. T., Ma, J. F., Di Marco, S. & Gallouzi, I.-E. Inducible nitric oxide synthase (iNOS) in muscle wasting syndrome, sarcopenia, and cachexia. Aging 3, 702–715 (2011).
Acharyya, S., Ladner, K. J., Nelsen, L. L., Damrauer, J., Reiser, P. J., Swoap, S. et al. Cancer cachexia is regulated by selective targeting of skeletal muscle gene products. J. Clin. Invest. 114, 370–378 (2004).
Gilabert, M., Calvo, E., Airoldi, A., Hamidi, T., Moutardier, V., Turrini, O. et al. Pancreatic cancer-induced cachexia Is Jak2-dependent in mice. J. Cell Physiol. 229, 1437–1443 (2014).
Zimmers, T. A., Fishel, M. L. & Bonetto, A. STAT3 in the systemic inflammation of cancer cachexia. Semin. Cell. Dev. Biol. 54, 28–41 (2016).
Lira, F. S., Yamashita, A. S., Rosa, J. C., Tavares, F. L., Caperuto, E., Carnevali, L. C. Jr. et al. Hypothalamic inflammation is reversed by endurance training in anorectic-cachectic rats. Nutr. Metab. 8, 60–60 (2011).
Plata-Salamán, C. R., Ilyin, S. E. & Gayle, D. Brain cytokine mRNAs in anorectic rats bearing prostate adenocarcinoma tumor cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 275, R566–R573 (1998).
Inui, A. & Neuropeptide, Y. a key molecule in anorexia and cachexia in wasting disorders? Mol. Med. Today 5, 79–85 (1999).
Reyes, T. M. & Sawchenko, P. E. Involvement of the arcuate nucleus of the hypothalamus in interleukin-1-induced anorexia. J. Neurosci. 22, 5091–5099 (2002).
Amitani, M., Asakawa, A., Amitani, H. & Inui, A. Control of food intake and muscle wasting in cachexia. Int. J. Biochem. Cell Biol. 45, 2179–2185 (2013).
Ji, Y. B., Bo, C. L., Xue, X. J., Weng, E. M., Gao, G. C., Dai, B. B. et al. Association of inflammatory cytokines with the symptom cluster of pain, fatigue, depression, and sleep disturbance in chinese patients with cancer. J. Pain Symptom Manage 54, 843–852 (2017).
Breitbart, W., Rosenfeld, B., Tobias, K., Pessin, H., Ku, G. Y., Yuan, J. et al. Depression, cytokines, and pancreatic cancer. Psychooncology 23, 339–345 (2014).
Yaskin, J. C. Nervous symptoms as earliest manifestations of carcinoma of the pancreas. JAMA 96, 1664–1668 (1931).
Morley, J. E. Anorexia of aging: physiologic and pathologic. Am. J. Clin. Nutr. 66, 760–773 (1997).
Moo-Young, T. A., Larson, J. W., Belt, B. A., Tan, M. C., Hawkins, W. G., Eberlein, T. J. et al. Tumor-derived TGF-beta mediates conversion of CD4+Foxp3+ regulatory T cells in a murine model of pancreas cancer. J. Immunother. 32, 12–21 (2009).
Löhr, M., Schmidt, C., Ringel, J., Kluth, M., Müller, P., Nizze, H. et al. Transforming Growth Factor-β1 induces desmoplasia in an experimental model of human pancreatic carcinoma. Cancer Res. 61, 550–555 (2001).
Zugmaier, G., Paik, S., Wilding, G., Knabbe, C., Bano, M., Lupu, R. et al. Transforming Growth Factor β1 induces cachexia and szystemic fibrosis without an antitumor effect in nude mice. Cancer Res. 51, 3590–3594 (1991).
Mendias, C. L., Gumucio, J. P., Davis, M. E., Bromley, C. W., Davis, C. S. & Brooks, S. V. Transforming growth factor-beta induces skeletal muscle atrophy and fibrosis through the induction of atrogin-1 and scleraxis. Muscle Nerve 45, 55–59 (2012).
Greco, S. H., Tomkotter, L., Vahle, A. K., Rokosh, R., Avanzi, A., Mahmood, S. K. et al. TGF-beta blockade reduces mortality and metabolic changes in a validated murine model of pancreatic cancer cachexia. PLoS ONE 10, e0132786 (2015).
Zimmers, T. A., Davies, M. V., Koniaris, L. G., Haynes, P., Esquela, A. F., Tomkinson, K. N. et al. Induction of cachexia in mice by systemically administered myostatin. Science 296, 1486–1488 (2002).
Chen, J. L., Walton, K. L., Winbanks, C. E., Murphy, K. T., Thomson, R. E., Makanji, Y. et al. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J. 28, 1711–1723 (2014).
Zhong, X., Pons, M., Poirier, C., Jiang, Y., Liu, J., Sandusky, G. E. et al. The systemic activin response to pancreatic cancer: implications for effective cancer cachexia therapy. J. Cachexia Sarcopenia Muscle 10, 1083–1101 (2019).
Koopmann, J., Buckhaults, P., Brown, D. A., Zahurak, M. L., Sato, N., Fukushima, N. et al. Serum macrophage inhibitory cytokine 1 as a marker of pancreatic and other periampullary cancers. Clin. Cancer Res. 10, 2386–2392 (2004).
Johnen, H., Lin, S., Kuffner, T., Brown, D. A., Tsai, V. W., Bauskin, A. R. et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 13, 1333–1340 (2007).
Todorov, P., Cariuk, P., McDevitt, T., Coles, B., Fearon, K. & Tisdale, M. Characterization of a cancer cachectic factor. Nature 379, 739–742 (1996).
Whitehouse, A. S. & Tisdale, M. J. Increased expression of the ubiquitin-proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-kappaB. Br. J. Cancer 89, 1116–1122 (2003).
Wyke, S. M. & Tisdale, M. J. NF-κB mediates proteolysis-inducing factor induced protein degradation and expression of the ubiquitin–proteasome system in skeletal muscle. Br. J. Cancer 92, 711–721 (2005).
Eley, H. L. & Tisdale, M. J. Skeletal muscle atrophy, a link between depression of protein synthesis and increase in degradation. J. Biol. Chem. 282, 7087–7097 (2007).
Wigmore, S. J., Todorov, P. T., Barber, M. D., Ross, J. A., Tisdale, M. J. & Fearon, K. C. H. Characteristics of patients with pancreatic cancer expressing a novel cancer cachectic factor. Br. J. Surg. 87, 53–58 (2000).
Watchorn, T. M., Waddell, I. D., Dowidar, N. & Ross, J. A. Proteolysis-inducing factor regulates hepatic gene expression via the transcription factors NF-κΒ and STAT3. FASEB J. 15, 562–564 (2001).
Huang, X.-Y., Huang, Z.-L., Yang, J.-H., Xu, Y.-H., Sun, J.-S., Zheng, Q. et al. Pancreatic cancer cell-derived IGFBP-3 contributes to muscle wasting. J Exp. Clin. Cancer Res. 35, 46 (2016).
He, W. A., Calore, F., Londhe, P., Canella, A., Guttridge, D. C. & Croce, C. M. Microvesicles containing miRNAs promote muscle cell death in cancer cachexia via TLR7. Proc. Natl Acad. Sci USA 111, 4525–4529 (2014).
Zhang, G., Liu, Z., Ding, H., Zhou, Y., Doan, H. A., Sin, K. W. T. et al. Tumor induces muscle wasting in mice through releasing extracellular Hsp70 and Hsp90. Nat. Commun 8, 589 (2017)
Sikkens, E. C. M., Cahen, D. L., de Wit, J., Looman, C. W. N., van Eijck, C. & Bruno, M. J. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J. Clin. Gastroenterol. 48, e43–e46 (2014).
Permert, J., Ihse, I., Jorfeldt, L., von Schenck, H., Arnqvist, H. J. & Larsson, J. Pancreatic cancer is associated with impaired glucose metabolism. Eur. J. Surg. 159, 101–107 (1993).
Lim, P.-W., Dinh, K. H., Sullivan, M., Wassef, W. Y., Zivny, J., Whalen, G. F. et al. Thirty-day outcomes underestimate endocrine and exocrine insufficiency after pancreatic resection. HPB (Oxford) 18, 360–366 (2016).
Maignan, A., Ouaissi, M., Turrini, O., Regenet, N., Loundou, A., Louis, G. et al. Risk factors of exocrine and endocrine pancreatic insufficiency after pancreatic resection: A multi-center prospective study. J. Visc. Surg. 155, 173–181 (2018).
Speicher, J. E. & Traverso, L. W. Pancreatic exocrine function is preserved after distal pancreatectomy. J. Gastroenterol. Surg. 14, 1006–1011 (2010).
Beger, H. G., Poch, B., Mayer, B. & Siech, M. New onset of diabetes and pancreatic exocrine insufficiency after pancreaticoduodenectomy for benign and malignant tumors. Ann. Surg. 267, 259–270 (2018).
Kang, M. J., Jung, H. S., Jang, J.-Y., Jung, W., Chang, J., Shin, Y. C. et al. Metabolic effect of pancreatoduodenectomy: resolution of diabetes mellitus after surgery. Pancreatology 16, 272–277 (2016).
Wu, J.-M., Kuo, T.-C., Yang, C.-Y., Chiang, P.-Y., Jeng, Y.-M., Huang, P.-H. et al. Resolution of diabetes after pancreaticoduodenectomy in patients with and without pancreatic ductal cell adenocarcinoma. Ann. Surg. Oncol. 20, 242–249 (2013).
Vujasinovic, M., Valente, R., Del Chiaro, M., Permert, J. & Löhr, J.-M. Pancreatic exocrine insufficiency in pancreatic cancer. Nutrients 9, 183 (2017).
Schober, M., Jesenofsky, R., Faissner, R., Weidenauer, C., Hagmann, W., Michl, P. et al. Desmoplasia and chemoresistance in pancreatic cancer. Cancers (Basel) 6, 2137–2154 (2014).
Brune, K., Abe, T., Canto, M., O’Malley, L., Klein, A. P., Maitra, A. et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am. J. Surg. Pathol. 30, 1067–1076 (2006).
Anagnostides, A., Chadwick, V., Selden, A. & Maton, P. Sham feeding and pancreatic secretion: evidence for direct vagal stimulation of enzyme output. Gastroenterology 87, 109–114 (1984).
White, T., McAlexander, R. & Magee, D. The effect of gastric distension on duodenal aspirates in man. Gastroenterology 44, 48–51 (1963).
Watanabe, S., Shiratori, K., Takeuchi, T., Chey, W., You, C. & Chang, T.-M. Release of cholecystokinin and exocrine pancreatic secretion in response to an elemental diet in human subjects. Dig. Dis. Sci. 31, 919–924 (1986).
Bapat, A. A., Hostetter, G., Von Hoff, D. D. & Han, H. Perineural invasion and associated pain in pancreatic cancer. Nat. Rev. Cancer. 11, 695–707 (2011).
Ceyhan, G. O., Demir, I. E., Rauch, U., Bergmann, F., Muller, M. W., Buchler, M. W. et al. Pancreatic neuropathy results in “neural remodeling” and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am. J. Gastroenterol. 104, 2555–2565 (2009).
Körner, M., Hayes, G. M., Rehmann, R., Zimmermann, A., Friess, H., Miller, L. J. et al. Secretin receptors in normal and diseased human pancreas: marked reduction of receptor binding in ductal neoplasia. Am. J. Patho. 167, 959–968 (2005).
Weinberg, D. S., Ruggeri, B., Barber, M. T., Biswas, S., Miknyocki, S., Waldman, S. A. & Cholecystokinin, A. and B receptors are differentially expressed in normal pancreas and pancreatic adenocarcinoma. J. Clin. Invest. 100, 597–603 (1997).
Weinberg, D. S., Heyt, G. J., Cavanagh, M., Pitchon, D., McGlynn, K. A. & London, W. T. Cholecystokinin and gastrin levels are not elevated in human pancreatic adenocarcinoma. Cancer Epidemiol. Biomarkers Prev. 10, 721–722 (2001).
Ji, B., Bi, Y., Simeone, D., Mortensen, R. M. & Logsdon, C. D. Human pancreatic acinar cells lack functional responses to cholecystokinin and gastrin. Gastroenterology 121, 1380–1390 (2001).
Shintakuya, R., Uemura, K., Murakami, Y., Kondo, N., Nakagawa, N., Urabe, K. et al. Sarcopenia is closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease. Pancreatology 17, 70–75 (2017).
Danai, L. V., Babic, A., Rosenthal, M. H., Dennstedt, E. A., Muir, A., Lien, E. C. et al. Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature 558, 600–604 (2018).
Gooden, H. M. & White, K. J. Pancreatic cancer and supportive care—pancreatic exocrine insufficiency negatively impacts on quality of life. Support Care Cancer 21, 1835–1841 (2013).
Zuijdgeest-Van Leeuwen, S. D., Van Der Heijden, M. S., Rietveld, T., Van Den Berg, J. W. O., Tilanus, H. W., Burgers, J. A. et al. Fatty acid composition of plasma lipids in patients with pancreatic, lung and oesophageal cancer in comparison with healthy subjects. Clin. Nutr. 21, 225–230 (2002).
Matejcic, M., Lesueur, F., Biessy, C., Renault, A. L., Mebirouk, N., Yammine, S. et al. Circulating plasma phospholipid fatty acids and risk of pancreatic cancer in a large European cohort. Int. J. Cancer 143, 2437–2448 (2018).
Murphy, R. A., Yeung, E., Mazurak, V. C. & Mourtzakis, M. Influence of eicosapentaenoic acid supplementation on lean body mass in cancer cachexia. Br. J. Cancer 105, 1469–1473 (2011).
Dewey, A., Baughan, C., Dean, T. P., Higgins, B. & Johnson I. Eicosapentaenoic acid (EPA, an omega‐3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst. Rev. CD004597 (2007)
Haaber, A. B., Rosenfalck, A. M., Hansen, B., Hilsted, J. & Larsen, S. Bone mineral metabolism, bone mineral density, and body composition in patients with chronic pancreatitis and pancreatic exocrine insufficiency. Int. J. Pancreatol. 27, 21–27 (2000).
Nakamura, T., Takebe, K., Imamura, K., Tando, Y., Yamada, N., Arai, Y. et al. Fat-soluble vitamins in patients with chronic pancreatitis (pancreatic insufficiency). Acta Gastroenterol. Belg. 59, 10–14 (1996).
Klapdor, S., Richter, E. & Klapdor, R. Vitamin D status and per-oral vitamin D supplementation in patients suffering from chronic pancreatitis and pancreatic cancer disease. Anticancer Res. 32, 1991–1998 (2012).
Dev, R., Del Fabbro, E., Schwartz, G. G., Hui, D., Palla, S. L., Gutierrez, N. et al. Preliminary report: vitamin D deficiency in advanced cancer patients with symptoms of fatigue or anorexia. Oncologist 16, 1637–1641 (2011).
Garcia, M., Seelaender, M., Sotiropoulos, A., Coletti, D. & Lancha, A. H. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition 60, 66–69 (2019).
Camperi, A., Pin, F., Costamagna, D., Penna, F., Menduina, M. L., Aversa, Z. et al. Vitamin D and VDR in cancer cachexia and muscle regeneration. Oncotarget 8, 21778–21793 (2017).
Mantovani, G., Madeddu, C. & Maccio, A. Cachexia and oxidative stress in cancer: an innovative therapeutic management. Curr. Pharm. Des. 18, 4813–4818 (2012).
Huang, B. Z., Pandol, S. J., Jeon, C. Y., Chari, S. T., Sugar, C. A., Chao, C. R. et al. New-onset diabetes, longitudinal trends in metabolic markers, and risk of pancreatic cancer in a heterogeneous population. J. Gastroenterol. Hepatol. 18, 1812–1821.e7 (2019).
Sah, R. P., Nagpal, S. J. S., Mukhopadhyay, D. & Chari, S. T. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat. Rev. Gastroenterol. Hepatol. 10, 423–433 (2013).
Aggarwal, G., Ramachandran, V., Javeed, N., Arumugam, T., Dutta, S., Klee, G. G. et al. Adrenomedullin is up-regulated in patients with pancreatic cancer and causes insulin resistance in beta cells and mice. Gastroenterology 143, 1510–1517.e1511 (2012).
Armstrong, E. A., Beal, E. W., Chakedis, J., Paredes, A. Z., Moris, D., Pawlik, T. M. et al. Exosomes in Pancreatic Cancer: from Early Detection to Treatment. J. Gastrointest. Surg. 22, 737–750 (2018).
Wang, W. S., Liu, X. H., Liu, L. X., Jin, D. Y., Yang, P. Y. & Wang, X. L. Identification of proteins implicated in the development of pancreatic cancer-associated diabetes mellitus by iTRAQ-based quantitative proteomics. J. Proteomics 84, 52–60 (2013).
Basso, D., Greco, E., Fogar, P., Pucci, P., Flagiello, A., Baldo, G. et al. Pancreatic cancer-associated diabetes mellitus: an open field for proteomic applications. Clin. Chim. Acta 357, 184–189 (2005).
Basso, D., Greco, E., Fogar, P., Pucci, P., Flagiello, A., Baldo, G. et al. Pancreatic cancer-derived S-100A8 N-terminal peptide: a diabetes cause? Clin. Chim. Acta 372, 120–128 (2006).
Permert, J., Larsson, J., Westermark, G. T., Herrington, M. K., Christmanson, L., Pour, P. M. et al. Islet amyloid polypeptide in patients with pancreatic cancer and diabetes. N. Engl. J. Med. 330, 313–318 (1994).
Ding, X., Flatt, P. R., Permert, J. & Adrian, T. E. Pancreatic cancer cells selectively stimulate islet β cells to secrete amylin. Gastroenterology 114, 130–138 (1998).
Liu, J., Knezetic, J. A., Strömmer, L., Permert, J., Larsson, J. R. & Adrian, T. E. The intracellular mechanism of insulin resistance in pancreatic cancer patients. J. Clin. Endocrinol. Metab. 85, 1232–1238 (2000).
Yoshikawa, T., Noguchi, Y., Doi, C., Makino, T., Okamoto, T. & Matsumoto, A. Insulin resistance was connected with the alterations of substrate utilization in patients with cancer. Cancer Lett. 141, 93–98 (1999).
Watanapa, P. & Williamson, R. C. Surgical palliation for pancreatic cancer: developments during the past two decades. Br. J. Surg. 79, 8–20 (1992).
Wong, Y. T., Brams, D. M., Munson, L., Sanders, L., Heiss, F., Chase, M. et al. Gastric outlet obstruction secondary to pancreatic cancer: surgical vs endoscopic palliation. Surgical Endosc. 16, 310–312 (2002).
Dzutsev, A., Badger, J. H., Perez-Chanona, E., Roy, S., Salcedo, R., Smith, C. K. et al. Microbes and cancer. Annu. Rev. Immunol. 35, 199–228 (2017).
Torres, P. J., Fletcher, E. M., Gibbons, S. M., Bouvet, M., Doran, K. S. & Kelley, S. T. Characterization of the salivary microbiome in patients with pancreatic cancer. Peer J. 3, e1373 (2015).
Farrell, J. J., Zhang, L., Zhou, H., Chia, D., Elashoff, D., Akin, D. et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 61, 582–588 (2012).
Pushalkar, S., Hundeyin, M., Daley, D., Zambirinis, C. P., Kurz, E., Mishra, A. et al. The pancreatic cancer microbiome promotes oncogenesis by induction of innate and adaptive immune suppression. Cancer Discov. 8, 403–416 (2018).
Sethi, V., Kurtom, S., Tarique, M., Lavania, S., Malchiodi, Z., Hellmund, L. et al. Gut microbiota promotes tumor growth in mice by modulating immune response. Gastroenterology 155, 33–37 e36 (2018).
Geller, L. T., Barzily-Rokni, M., Danino, T., Jonas, O. H., Shental, N., Nejman, D. et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science 357, 1156–1160 (2017).
Li, S., Fuhler, G. M., Bn, N., Jose, T., Bruno, M. J., Peppelenbosch, M. P. et al. Pancreatic cyst fluid harbors a unique microbiome. Microbiome 5, 147 (2017).
Bindels, L. B., Neyrinck, A. M., Loumaye, A., Catry, E., Walgrave, H., Cherbuy, C. et al. Increased gut permeability in cancer cachexia: mechanisms and clinical relevance. Oncotarget 9, 18224–18238 (2018).
Pötgens, S. A., Brossel, H., Sboarina, M., Catry, E., Cani, P. D., Neyrinck, A. M. et al. Klebsiella oxytoca expands in cancer cachexia and acts as a gut pathobiont contributing to intestinal dysfunction. Sci. Rep. 8, 12321 (2018).
Bures, J., Cyrany, J., Kohoutova, D., Forstl, M., Rejchrt, S., Kvetina, J. et al. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 16, 2978–2990 (2010).
Nishiyama, H., Nagai, T., Kudo, M., Okazaki, Y., Azuma, Y., Watanabe, T. et al. Supplementation of pancreatic digestive enzymes alters the composition of intestinal microbiota in mice. Biochem. Biophys. Res. Commun. 495, 273–279 (2018).
Varian, B. J., Gourishetti, S., Poutahidis, T., Lakritz, J. R., Levkovich, T., Kwok, C. et al. Beneficial bacteria inhibit cachexia. Oncotarget 7, 11803–11816 (2016).
Routy, B., Le Chatelier, E., Derosa, L., Duong, C. P. M., Alou, M. T., Daillère, R. et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359, 91–97 (2018).
Ahuja, M., Schwartz, D. M., Tandon, M., Son, A., Zeng, M., Swaim, W. et al. Orai1-mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut innate immunity. Cell Metab. 25, 635–646 (2017).
Nay, K., Jollet, M., Goustard, B., Baati, N., Vernus, B., Pontones, M. et al. Gut bacteria are critical for optimal muscle function: a potential link with glucose homeostasis. Am. J. Physiol. Endocrinol. Metab. 317, E158–E171 (2019).
Oliphant, K. & Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7, 91 (2019).
Liu, R., Hong, J., Xu, X., Feng, Q., Zhang, D., Gu, Y. et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23, 859–868 (2017).
Biswas, A. K. & Acharyya, S. Understanding cachexia in the context of metastatic progression. Nat. Rev. Cancer 20, 274–284 (2020).
Yakovenko, A., Cameron, M. & Trevino, J. G. Molecular therapeutic strategies targeting pancreatic cancer induced cachexia. World J. Gastrointest. Surg. 10, 95–106 (2018).
Arends, J., Bachmann, P., Baracos, V., Barthelemy, N., Bertz, H., Bozzetti, F. et al. ESPEN guidelines on nutrition in cancer patients. Clin. Nutr. 36, 11–48 (2017).
Shukla, S. K., Gebregiworgis, T., Purohit, V., Chaika, N. V., Gunda, V., Radhakrishnan, P. et al. Metabolic reprogramming induced by ketone bodies diminishes pancreatic cancer cachexia. Cancer Metab. 2, 18 (2014).
May, P. E., Barber, A., D’Olimpio, J. T., Hourihane, A. & Abumrad, N. N. Reversal of cancer-related wasting using oral supplementation with a combination of β-hydroxy-β-methylbutyrate, arginine, and glutamine. Am. J. Surg. 183, 471–479 (2002).
Holecek, M. Side effects of long-term glutamine supplementation. JPEN J. Parenter Enteral. Nutr. 37, 607–616 (2012).
Smith, H. J., Mukerji, P. & Tisdale, M. J. Attenuation of proteasome-induced proteolysis in skeletal muscle by β-hydroxy-β-methylbutyrate in cancer-induced muscle loss. Cancer Res. 65, 277–283 (2005).
Wilkinson, D. J., Hossain, T., Hill, D. S., Phillips, B. E., Crossland, H., Williams, J. et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 591, 2911–2923 (2013).
Eley Helen, L., Russell Steven, T., Tisdale & Michael, J. Effect of branched-chain amino acids on muscle atrophy in cancer cachexia. Biochem. J. 407, 113–120 (2007).
Tayek, J. A., Bistrian, B. R., Hehir, D. J., Martin, R., Moldawer, L. L. & Blackburn, G. L. Improved protein kinetics and albumin synthesis by branched chain amino acid‐enriched total parenteral nutrition in cancer cachexia: a prospective randomized crossover trial. Cancer 58, 147–157 (1986).
Deutz, N. E., Safar, A., Schutzler, S., Memelink, R., Ferrando, A., Spencer, H. et al. Muscle protein synthesis in cancer patients can be stimulated with a specially formulated medical food. Clin. Nutr. 30, 759–768 (2011).
Berk, L., James, J., Schwartz, A., Hug, E., Mahadevan, A., Samuels, M. et al. A randomized, double-blind, placebo-controlled trial of a beta-hydroxyl beta-methyl butyrate, glutamine, and arginine mixture for the treatment of cancer cachexia (RTOG 0122). Support Care Cancer 16, 1179–1188 (2008).
Malta, F. A. P. S., Estadella, D. & Gonçalves, D. C. The role of omega 3 fatty acids in suppressing muscle protein catabolism: a possible therapeutic strategy to reverse cancer cachexia? J. Funct. Foods 54, 1–12 (2019).
Wigmore, S. J., Barber, M. D., Ross, J. A., Tisdale, M. J. & Fearon, K. C. H. Effect of oral eicosapentaenoic acid on weight loss in patients with pancreatic cancer. Nutr Cancer 36, 177–184 (2000).
Abe, K., Uwagawa, T., Haruki, K., Takano, Y., Onda, S., Sakamoto, T. et al. Effects of ω-3 fatty acid supplementation in patients with bile duct or pancreatic cancer undergoing chemotherapy. Anticancer Res. 38, 2369–2375 (2018).
Fearon, K. C. H., von Meyenfeldt, M. F., Moses, A. G. W., van Geenen, R., Roy, A., Gouma, D. J. et al. Effect of a protein and energy dense n-3 fatty acid enriched oral supplement on loss of weight and lean tissue in cancer cachexia: a randomised double blind trial. Gut 52, 1479–1486 (2003).
Hinson, R. M., Williams, J. A. & Shacter, E. Elevated interleukin 6 is induced by prostaglandin E2 in a murine model of inflammation: possible role of cyclooxygenase-2. Proc. Natl Acad. Sci. USA 93, 4885–4890 (1996).
Kunkel, S. L., Spengler, M., May, M. A., Spengler, R., Larrick, J. & Remick, D. Prostaglandin E2 regulates macrophage-derived tumor necrosis factor gene expression. J. Biol. Chem. 263, 5380–5384 (1988).
Thompson, M. G., Pascal, M., Mackie, S. C., Thom, A., Morrison, K. S., Colette Backwell, F. R. et al. Evidence that protein kinase C and mitogen activated protein kinase are not involved in the mechanism by which insulin stimulates translation in L6 myoblasts. Biosci. Rep. 15, 37–46 (1995).
Mantovani, G., Maccio, A., Madeddu, C., Serpe, R., Antoni, G., Massa, E. et al. Phase II nonrandomized study of the efficacy and safety of COX-2 inhibitor celecoxib on patients with cancer cachexia. J. Mol. Med. (Berl) 88, 85–92 (2010).
Lai, V., George, J., Richey, L., Kim, H. J., Cannon, T., Shores, C. et al. Results of a pilot study of the effects of celecoxib on cancer cachexia in patients with cancer of the head, neck, and gastrointestinal tract. Head Neck 30, 67–74 (2008).
McMillan, D. C., Wigmore, S. J., Wigmore, K. C. H., O’Gorman, P., Wright, C. E. & McArdle, C. S. A prospective randomized study of megestrol acetate and ibuprofen in gastrointestinal cancer patients with weight loss. Br J Cancer 79, 495–500 (1999).
McMillan, D. C., Simpson, J. M., Preston, T., Watson, W. S., Fearon, K. C. H., Shenkin, A. et al. Effect of megestrol acetate on weight loss, body composition and blood screen of gastrointestinal cancer patients. Clin. Nutr. 13, 85–89 (1994).
Gordon, J. N., Trebble, T. M., Ellis, R. D., Duncan, H. D., Johns, T. & Goggin, P. M. Thalidomide in the treatment of cancer cachexia: a randomised placebo controlled trial. Gut 54, 540–545 (2005).
Wiedenmann, B., Malfertheiner, P., Friess, H., Ritch, P., Arseneau, J., Mantovani, G. et al. A multicenter, phase II study of infliximab plus gemcitabine in pancreatic cancer cachexia. J. Support. Oncol. 6, 18–25 (2008).
Hurwitz, H., Van Cutsem, E., Bendell, J., Hidalgo, M., Li, C.-P., Salvo, M. G. et al. Ruxolitinib + capecitabine in advanced/metastatic pancreatic cancer after disease progression/intolerance to first-line therapy: JANUS 1 and 2 randomized phase III studies. Invest. New Drugs 36, 683–695 (2018).
Solheim, T. S., Laird, B. J. A., Balstad, T. R., Stene, G. B., Bye, A., Johns, N. et al. A randomized phase II feasibility trial of a multimodal intervention for the management of cachexia in lung and pancreatic cancer. J. Cachexia Sarcopenia Muscle 8, 778–788 (2017).
Solheim, T. S., Laird, B. J., Balstad, T. R., Bye, A., Stene, G., Baracos, V. et al. Cancer cachexia: rationale for the MENAC (Multimodal—Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support. Palliat. Care 8, 258–265 (2018).
Wiskemann, J., Clauss, D., Tjaden, C., Hackert, T., Schneider, L., Ulrich, C. M. et al. Progressive resistance training to impact physical fitness and body weight in pancreatic cancer patients: A randomized controlled trial. Pancreas 48, 257–266 (2019).
Hamauchi, S., Furuse, J., Takano, T., Munemoto, Y., Furuya, K., Baba, H. et al. A multicenter, open-label, single-arm study of anamorelin (ONO-7643) in advanced gastrointestinal cancer patients with cancer cachexia. Cancer 125, 4294–4302 (2019).
Koch, M., Varela, L., Kim, J. G., Kim, J. D., Hernández-Nuño, F., Simonds, S. E. et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature 519, 45–50 (2015).
Jatoi, A., Windschitl, H. E., Loprinzi, C. L., Sloan, J. A., Dakhil, S. R., Mailliard, J. A. et al. Dronabinol versus megestrol acetate versus combination therapy for cancer-associated anorexia: a North Central Cancer Treatment Group study. J. Clin. Oncol. 20, 567–573 (2002).
Golan, T., Geva, R., Richards, D., Madhusudan, S., Lin, B. K., Wang, H. T. et al. LY2495655, an antimyostatin antibody, in pancreatic cancer: a randomized, phase 2 trial. J. Cachexia Sarcopenia Muscle 9, 871–879 (2018).
Riechelmann, R. P., Burman, D., Tannock, I. F., Rodin, G. & Zimmermann, C. Phase II trial of mirtazapine for cancer-related cachexia and anorexia. Am J Hosp. Palliat. Med. 27, 106–110 (2010).
Landers, A., Muircroft, W. & Brown, H. Pancreatic enzyme replacement therapy (PERT) for malabsorption in patients with metastatic pancreatic cancer. BMJ Support. Palliat. Care 6, 75–79 (2016).
Bruno, M., Haverkort, E., Tijssen, G., Tytgat, G., Van & Leeuwen, D. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut 42, 92–96 (1998).
Dominguez-Munoz, J. E., Nieto-Garcia, L., Lopez-Diaz, J., Larino-Noia, J., Abdulkader, I. & Iglesias-Garcia, J. Impact of the treatment of pancreatic exocrine insufficiency on survival of patients with unresectable pancreatic cancer: a retrospective analysis. BMC Cancer 18, 534 (2018).
Woo, S. M., Joo, J., Kim, S. Y., Park, S. J., Han, S. S., Kim, T. H. et al. Efficacy of pancreatic exocrine replacement therapy for patients with unresectable pancreatic cancer in a randomized trial. Pancreatology 16, 1099–1105 (2016).
Iglesia, D. D. L., Avci, B., Kiriukova, M., Panic, N., Bozhychko, M., Sandru, V. et al. Pancreatic exocrine insufficiency and pancreatic enzyme replacement therapy in patients with advanced pancreatic cancer: a systematic review and meta-analysis. United European Gastroenterol. J. 8, 1115–1125 (2020).
Johnson, C. Guidelines for the management of patients with pancreatic cancer periampullary and ampullary carcinomas. Gut 54, v1–v16 (2005).
Tempero, M. A., Malafa, M. P., Al-Hawary, M., Asbun, H., Bain, A., Behrman, S. W. et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Canc Netw 15, 1028–1061 (2017).
Löhr, J. M., Dominguez-Munoz, E., Rosendahl, J., Besselink, M., Mayerle, J., Lerch, M. M. et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol. J. 5, 153–199 (2017).
Andersen, D. K., Korc, M., Petersen, G. M., Eibl, G., Li, D., Rickels, M. R. et al. Diabetes, pancreatogenic diabetes, and pancreatic cancer. Diabetes 66, 1103–1110 (2017).
Madiraju, A. K., Erion, D. M., Rahimi, Y., Zhang, X. M., Braddock, D. T., Albright, R. A. et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature 510, 542–546 (2014).
Oliveira, A. G. & Gomes-Marcondes, M. C. C. Metformin treatment modulates the tumour-induced wasting effects in muscle protein metabolism minimising the cachexia in tumour-bearing rats. BMC Cancer 16, 418 (2016).
Kordes, S., Pollak, M. N., Zwinderman, A. H., Mathôt, R. A., Weterman, M. J., Beeker, A. et al. Metformin in patients with advanced pancreatic cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 16, 839–847 (2015).
Chen, S.-M., Chieng, W.-W., Huang, S.-W., Hsu, L.-J. & Jan, M.-S. The synergistic tumor growth-inhibitory effect of probiotic Lactobacillus on transgenic mouse model of pancreatic cancer treated with gemcitabine. Sci. Rep. 10, 20319 (2020).
Riquelme, E., Zhang, Y., Zhang, L., Montiel, M., Zoltan, M., Dong, W. et al. Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell 178, 795–806.e712 (2019).
Cheng, W. Y., Wu, C.-Y. & Yu, J. The role of gut microbiota in cancer treatment: friend or foe? Gut 69, 1867–1876 (2020).
Hingorani, S. R., Wang, L., Multani, A. S., Combs, C., Deramaudt, T. B., Hruban, R. H. et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell 7, 469–483 (2005).
Aguirre, A. J., Bardeesy, N., Sinha, M., Lopez, L., Tuveson, D. A., Horner, J. et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 17, 3112–3126 (2003).
Talbert, E. E., Cuitiño, M. C., Ladner, K. J., Rajasekerea, P. V., Siebert, M., Shakya, R. et al. Modeling human cancer-induced cachexia. Cell Rep. 28, 1612–1622.e1614 (2019).
Henderson, S. E., Makhijani, N. & Mace, T. A. Pancreatic cancer–induced cachexia and relevant mouse models. Pancreas 47, 937–945 (2018).
Acknowledgements
The authors would like to thank Rainer Heuchel for his critical comments and Erika Nieser for language editing.
Author information
Authors and Affiliations
Contributions
Conception and design: M.K. and J.M.L. Manuscript writing: M.K. and J.M.L. Final approval of manuscript: all authors. Accountable for all aspects of the work: all authors.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent to publish
Not applicable.
Data availability
Not applicable.
Competing interests
M.K.: Alligator Bioscience (Consulting), Roche (Consulting); L.L.: no disclosures; L.E.: no disclosures; J.M.L.: Abbot (Honoraria), Mylan (Honoraria).
Funding information
This work was supported through a clinician-scientist grant from Region Stockholm to M.K. and the Lars Vesterlund minnesfond to J.M.L.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kordes, M., Larsson, L., Engstrand, L. et al. Pancreatic cancer cachexia: three dimensions of a complex syndrome. Br J Cancer 124, 1623–1636 (2021). https://doi.org/10.1038/s41416-021-01301-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-021-01301-4
This article is cited by
-
Effect of cancer cachexia on first-line chemotherapy in patients with advanced pancreatic cancer: a claims database study in Japan
International Journal of Clinical Oncology (2024)
-
S100A8, S100A9 and S100A8/A9 heterodimer as novel cachexigenic factors for pancreatic cancer-induced cachexia
BMC Cancer (2023)
-
Adipose tissue and skeletal muscle wasting precede clinical diagnosis of pancreatic cancer
Nature Communications (2023)
-
Pancreatic cancer: branched-chain amino acids as putative key metabolic regulators?
Cancer and Metastasis Reviews (2021)