Abstract
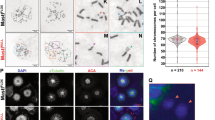
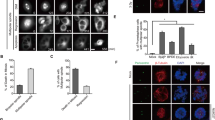
JunB, an activator protein-1 (AP-1) transcription factor component, acts either as a tumor suppressor or as an oncogene depending on the cell context. In particular, JunB is strongly upregulated in anaplastic lymphoma kinase (ALK)-positive anaplastic large cell lymphoma (ALCL) where it enhances cell proliferation. Although its overexpression is linked to lymphomagenesis, the mechanisms whereby JunB promotes neoplastic growth are still largely obscure. Here, we show that JunB undergoes coordinated phosphorylation-dependent ubiquitylation during the G2 phase of the cell cycle. We characterized a critical consensus phospho-degron that controls JunB turnover and identified GSK3 and SCFFBXW7 as, respectively, the kinase and the E3 ubiquitin ligase responsible for its degradation in G2. Pharmacological or genetic inactivation of the GSK3-FBXW7-JunB axis induced accumulation of JunB in G2/M and entailed transcriptional repression of the DNA helicase DDX11, leading to premature sister chromatid separation. This abnormal phenotype due to dysregulation of the GSK3β/JunB/DDX11 pathway is phenocopied in ALK-positive ALCL. Thus, our results reveal a novel mechanism by which mitosis progression and chromatid cohesion are regulated through GSK3/SCFFBXW7-mediated proteolysis of JunB, and suggest that JunB proteolysis in G2 is an essential step in maintaining genetic fidelity during mitosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Amin HM, Lai R . Pathobiology of ALK+ anaplastic large-cell lymphoma. Blood 2007; 110: 2259–2267.
Morris SW, Kirstein MN, Valentine MB, Dittmer KG, Shapiro DN, Saltman DL et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 1994; 263: 1281–1284.
Palmer RH, Vernersson E, Grabbe C, Hallberg B . Anaplastic lymphoma kinase: signalling in development and disease. Biochem J 2009; 420: 345–361.
Fadlelmola FM, Zhou M, de Leeuw RJ, Dosanjh NS, Harmer K, Huntsman D et al. Sub-megabase resolution tiling (SMRT) array-based comparative genomic hybridization profiling reveals novel gains and losses of chromosomal regions in Hodgkin lymphoma and anaplastic large cell lymphoma cell lines. Mol Cancer 2008; 7: 2.
Mussolin L, Pillon M, Bonato P, Leszl A, Franceschetto G, Di Meglio A et al. Cytogenetic analysis of pediatric anaplastic large cell lymphoma. Pediatr Blood Cancer 2010; 55: 446–451.
Nagel S, Leich E, Quentmeier H, Meyer C, Kaufmann M, Drexler HG et al. Amplification at 7q22 targets cyclin-dependent kinase 6 in T-cell lymphoma. Leukemia 2008; 22: 387–392.
Piccaluga PP, Gazzola A, Mannu C, Agostinelli C, Bacci F, Sabattini E et al. Pathobiology of anaplastic large cell lymphoma. Adv Hematol 2010. 345053.
Salaverria I, Bea S, Lopez-Guillermo A, Lespinet V, Pinyol M, Burkhardt B et al. Genomic profiling reveals different genetic aberrations in systemic ALK-positive and ALK-negative anaplastic large cell lymphomas. Br J Haematol 2008; 140: 516–526.
Zettl A, Rudiger T, Konrad MA, Chott A, Simonitsch-Klupp I, Sonnen R et al. Genomic profiling of peripheral T-cell lymphoma, unspecified, and anaplastic large T-cell lymphoma delineates novel recurrent chromosomal alterations. Am J Pathol 2004; 164: 1837–1848.
Staber PB, Vesely P, Haq N, Ott RG, Funato K, Bambach I et al. The oncoprotein NPM-ALK of anaplastic large-cell lymphoma induces JUNB transcription via ERK1/2 and JunB translation via mTOR signaling. Blood 2007; 110: 3374–3383.
Shaulian E, Karin M . AP-1 in cell proliferation and survival. Oncogene 2001; 20: 2390–2400.
Eferl R, Wagner EF . AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer 2003; 3: 859–868.
Lopez-Bergami P, Lau E, Ronai Z . Emerging roles of ATF2 and the dynamic AP1 network in cancer. Nat Rev Cancer 2010; 10: 65–76.
Piechaczyk M, Farras R . Regulation and function of JunB in cell proliferation. Biochem Soc Trans 2008; 36 (Pt 5): 864–867.
Passegué E, Jochum W, Schorpp-Kistner M, Mohle-Steinlein U, Wagner EF . Chronic myeloid leukemia with increased granulocyte progenitors in mice lacking junB expression in the myeloid lineage. Cell 2001; 104: 21–32.
Passegué E, Wagner EF, Weissman IL . JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 2004; 119: 431–443.
Bruchova H, Borovanova T, Klamova H, Brdicka R . Gene expression profiling in chronic myeloid leukemia patients treated with hydroxyurea. Leuk Lymphoma 2002; 43: 1289–1295.
Dorsam ST, Ferrell CM, Dorsam GP, Derynck MK, Vijapurkar U, Khodabakhsh D et al. The transcriptome of the leukemogenic homeoprotein HOXA9 in human hematopoietic cells. Blood 2004; 103: 1676–1684.
Bossy-Wetzel E, Bravo R, Hanahan D . Transcription factors junB and c-jun are selectively up-regulated and functionally implicated in fibrosarcoma development. Genes Dev 1992; 6: 2340–2351.
Li B, Tournier C, Davis RJ, Flavell RA . Regulation of IL-4 expression by the transcription factor JunB during T helper cell differentiation. EMBO J 1999; 18: 420–432.
Passegué E, Jochum W, Behrens A, Ricci R, Wagner EF . JunB can substitute for Jun in mouse development and cell proliferation. Nat Genet 2002; 30: 158–166.
Bakiri L, Lallemand D, Bossy-Wetzel E, Yaniv M . Cell cycle-dependent variations in c-Jun and JunB phosphorylation: a role in the control of cyclin D1 expression. EMBO J 2000; 19: 2056–2068.
Passegué E, Wagner EF . JunB suppresses cell proliferation by transcriptional activation of p16(INK4a) expression. EMBO J 2000; 19: 2969–2979.
Andrecht S, Kolbus A, Hartenstein B, Angel P, Schorpp-Kistner M . Cell cycle promoting activity of JunB through cyclin A activation. J Biol Chem 2002; 277: 35961–35968.
Farràs R, Baldin V, Gallach S, Acquaviva C, Bossis G, Jariel-Encontre I et al. JunB breakdown in mid-/late G2 is required for down-regulation of cyclin A2 levels and proper mitosis. Mol Cell Biol 2008; 28: 4173–4187.
Mathas S, Hinz M, Anagnostopoulos I, Krappmann D, Lietz A, Jundt F et al. Aberrantly expressed c-Jun and JunB are a hallmark of Hodgkin lymphoma cells, stimulate proliferation and synergize with NF-kappa B. Embo J 2002; 21: 4104–4113.
Rassidakis GZ, Thomaides A, Atwell C, Ford R, Jones D, Claret FX et al. JunB expression is a common feature of CD30+ lymphomas and lymphomatoid papulosis. Mod Pathol 2005; 18: 1365–1370.
Watanabe M, Sasaki M, Itoh K, Higashihara M, Umezawa K, Kadin ME et al. JunB induced by constitutive CD30-extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase signaling activates the CD30 promoter in anaplastic large cell lymphoma and reed-sternberg cells of Hodgkin lymphoma. Cancer Res 2005; 65: 7628–7634.
Mao X, Orchard G, Lillington DM, Russell-Jones R, Young BD, Whittaker SJ . Amplification and overexpression of JUNB is associated with primary cutaneous T-cell lymphomas. Blood 2003; 101: 1513–1519.
Fang D, Elly C, Gao B, Fang N, Altman Y, Joazeiro C et al. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat Immunol 2002; 3: 281–287.
Fuchs SY, Xie B, Adler V, Fried VA, Davis RJ, Ronai Z . c-Jun NH2-terminal kinases target the ubiquitination of their associated transcription factors. J Biol Chem 1997; 272: 32163–32168.
Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC et al. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. Science 2004; 306: 271–275.
Welcker M, Clurman BE . FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 2008; 8: 83–93.
Wei W, Jin J, Schlisio S, Harper JW, Kaelin WG . The v-Jun point mutation allows c-Jun to escape GSK3-dependent recognition and destruction by the Fbw7 ubiquitin ligase. Cancer Cell 2005; 8: 25–33.
Welcker M, Orian A, Jin J, Grim JE, Harper JW, Eisenman RN et al. The Fbw7 tumor suppressor regulates glycogen synthase kinase 3 phosphorylation-dependent c-Myc protein degradation. Proc Natl Acad Sci USA 2004; 101: 9085–9090.
Ye X, Nalepa G, Welcker M, Kessler BM, Spooner E, Qin J et al. Recognition of phosphodegron motifs in human cyclin E by the SCF(Fbw7) ubiquitin ligase. J Biol Chem 2004; 279: 50110–50119.
Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature 2011; 471: 104–109.
Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature 2011; 471: 110–114.
Aoki M, Batista O, Bellacosa A, Tsichlis P, Vogt PK . The akt kinase: molecular determinants of oncogenicity. Proc Natl Acad Sci USA 1998; 95: 14950–14955.
Farina A, Shin JH, Kim DH, Bermudez VP, Kelman Z, Seo YS et al. Studies with the human cohesin establishment factor, ChlR1. Association of ChlR1 with Ctf18-RFC and Fen1. J Biol Chem 2008; 283: 20925–20936.
Inoue A, Li T, Roby SK, Valentine MB, Inoue M, Boyd K et al. Loss of ChlR1 helicase in mouse causes lethality due to the accumulation of aneuploid cells generated by cohesion defects and placental malformation. Cell Cycle 2007; 6: 1646–1654.
Parish JL, Rosa J, Wang X, Lahti JM, Doxsey SJ, Androphy EJ . The DNA helicase ChlR1 is required for sister chromatid cohesion in mammalian cells. J Cell Sci 2006; 119 (Pt 23): 4857–4865.
Singh RR, Cho-Vega JH, Davuluri Y, Ma S, Kasbidi F, Milito C et al. Sonic hedgehog signaling pathway is activated in ALK-positive anaplastic large cell lymphoma. Cancer Res 2009; 69: 2550–2558.
Kovary K, Bravo R . The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol 1991; 11: 4466–4472.
Lallemand D, Spyrou G, Yaniv M, Pfarr CM . Variations in Jun and Fos protein expression and AP-1 activity in cycling, resting and stimulated fibroblasts. Oncogene 1997; 14: 819–830.
Rajagopalan H, Jallepalli PV, Rago C, Velculescu VE, Kinzler KW, Vogelstein B et al. Inactivation of hCDC4 can cause chromosomal instability. Nature 2004; 428: 77–81.
Babaei-Jadidi R, Li N, Saadeddin A, Spencer-Dene B, Jandke A, Muhammad B et al. FBXW7 influences murine intestinal homeostasis and cancer, targeting Notch, Jun, and DEK for degradation. J Exp Med 2011; 208: 295–312.
Barber TD, McManus K, Yuen KW, Reis M, Parmigiani G, Shen D et al. Chromatid cohesion defects may underlie chromosome instability in human colorectal cancers. Proc Natl Acad Sci USA 2008; 105: 3443–3448.
Peters JM, Tedeschi A, Schmitz J . The cohesin complex and its roles in chromosome biology. Genes Dev 2008; 22: 3089–3114.
van der Lelij P, Chrzanowska KH, Godthelp BC, Rooimans MA, Oostra AB, Stumm M et al. Warsaw breakage syndrome, a cohesinopathy associated with mutations in the XPD helicase family member DDX11/ChlR1. Am J Hum Genet 2010; 86: 262–266.
Kang T, Wei Y, Honaker Y, Yamaguchi H, Appella E, Hung MC et al. GSK-3 beta targets Cdc25A for ubiquitin-mediated proteolysis, and GSK-3 beta inactivation correlates with Cdc25A overproduction in human cancers. Cancer Cell 2008; 13: 36–47.
Crusio KM, King B, Reavie LB, Aifantis I . The ubiquitous nature of cancer: the role of the SCF(Fbw7) complex in development and transformation. Oncogene 2010; 29: 4865–4873.
Bossis G, Ferrara P, Acquaviva C, Jariel-Encontre I, Piechaczyk M . c-Fos proto-oncoprotein is degraded by the proteasome independently of its own ubiquitinylation in vivo. Mol Cell Biol 2003; 23: 7425–7436.
Acknowledgements
We thank Sandra Gallach and Pablo Mateos for their excellent technical support and A Ferrando, TM Thomson, G Bossis and O Coux for fruitful discussions and critical reading of the manuscript. This research was supported by grants from the Fondo de Investigaciones Sanitarias (PI08/1127) and from Valencia’s Regional Ministry of Health (AP007/11) to RF, from the Spanish Ministry of Science and Innovation (SAF2009-08334) to JFM. RF is supported by the Institute of Health Carlos III and by the Regional Ministry of Health. MP was supported by the program ‘Equipe Labellisée’ of the French Ligue against Cancer. We are grateful to B Vogelstein for providing DLD1 and DLD1FBXW7−/− cells, M Pagano for Flag-tagged SKP2, CDH1 and CDC20 constructs, O Sangfield for Flag-tagged FBXW7α and FBXW7γ constructs, C Bonne-Andrea for Flag-tagged FBXW7α(R465A), PK Vogt (Scripps Research Institute) for activated AKT and E Noguchi for DDX11.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Pérez-Benavente, B., García, J., Rodríguez, M. et al. GSK3-SCFFBXW7 targets JunB for degradation in G2 to preserve chromatid cohesion before anaphase. Oncogene 32, 2189–2199 (2013). https://doi.org/10.1038/onc.2012.235
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2012.235
Keywords
This article is cited by
-
New roles for AP-1/JUNB in cell cycle control and tumorigenic cell invasion via regulation of cyclin E1 and TGF-β2
Genome Biology (2022)
-
AP-1 family transcription factors: a diverse family of proteins that regulate varied cellular activities in classical hodgkin lymphoma and ALK+ ALCL
Experimental Hematology & Oncology (2021)
-
Truncation of the TPR domain of OGT alters substrate and glycosite selection
Analytical and Bioanalytical Chemistry (2021)
-
Regulation of cell cycle drivers by Cullin-RING ubiquitin ligases
Experimental & Molecular Medicine (2020)
-
The ubiquitin-like modifier FAT10 interferes with SUMO activation
Nature Communications (2019)