Abstract
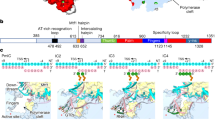
Human mitochondrial transcription factor A, TFAM, is essential for mitochondrial DNA packaging and maintenance and also has a crucial role in transcription. Crystallographic analysis of TFAM in complex with an oligonucleotide containing the mitochondrial light strand promoter (LSP) revealed two high-mobility group (HMG) protein domains that, through different DNA recognition properties, intercalate residues at two inverted DNA motifs. This induced an overall DNA bend of ~180°, stabilized by the interdomain linker. This U-turn allows the TFAM C-terminal tail, which recruits the transcription machinery, to approach the initiation site, despite contacting a distant DNA sequence. We also ascertained that structured protein regions contacting DNA in the crystal were highly flexible in solution in the absence of DNA. Our data suggest that TFAM bends LSP to create an optimal DNA arrangement for transcriptional initiation while facilitating DNA compaction elsewhere in the genome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
20 December 2011
In the version of this article initially published, the name of the second author was misspelled. The error has been corrected in the HTML and PDF versions of the article.
References
Brandon, M.C. et al. MITOMAP: a human mitochondrial genome database—2004 update. Nucleic Acids Res. 33 (Database issue), D611–3 (2005).
Fisher, R.P., Topper, J.N. & Clayton, D.A. Promoter selection in human mitochondria involves binding of a transcription factor to orientation-independent upstream regulatory elements. Cell 50, 247–258 (1987).
Montoya, J., Gaines, G.L. & Attardi, G. The pattern of transcription of the human mitochondrial rRNA genes reveals two overlapping transcription units. Cell 34, 151–159 (1983).
Legros, F., Malka, F., Frachon, P., Lombes, A. & Rojo, M. Organization and dynamics of human mitochondrial DNA. J. Cell Sci. 117, 2653–2662 (2004).
Iborra, F.J., Kimura, H. & Cook, P.R. The functional organization of mitochondrial genomes in human cells. BMC Biol. 2, 9 (2004).
Bogenhagen, D.F., Rousseau, D. & Burke, S. The layered structure of human mitochondrial DNA nucleoids. J. Biol. Chem. 283, 3665–3675 (2008).
Kaufman, B.A. et al. The mitochondrial transcription factor TFAM coordinates the assembly of multiple DNA molecules into nucleoid-like structures. Mol. Biol. Cell 18, 3225–3236 (2007).
Fisher, R.P., Lisowsky, T., Parisi, M.A. & Clayton, D.A. DNA wrapping and bending by a mitochondrial high mobility group-like transcriptional activator protein. J. Biol. Chem. 267, 3358–3367 (1992).
Ekstrand, M.I. et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 13, 935–944 (2004).
Kanki, T. et al. Architectural role of mitochondrial transcription factor A in maintenance of human mitochondrial DNA. Mol. Cell. Biol. 24, 9823–9834 (2004).
Larsson, N.G. et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat. Genet. 18, 231–236 (1998).
Takamatsu, C. et al. Regulation of mitochondrial D-loops by transcription factor A and single-stranded DNA-binding protein. EMBO Rep. 3, 451–456 (2002).
Alam, T.I. et al. Human mitochondrial DNA is packaged with TFAM. Nucleic Acids Res. 31, 1640–1645 (2003).
Ghivizzani, S.C., Madsen, C.S., Nelen, M.R., Ammini, C.V. & Hauswirth, W.W. In organello footprint analysis of human mitochondrial DNA: human mitochondrial transcription factor A interactions at the origin of replication. Mol. Cell. Biol. 14, 7717–7730 (1994).
Ohno, T., Umeda, S., Hamasaki, N. & Kang, D. Binding of human mitochondrial transcription factor A, an HMG box protein, to a four-way DNA junction. Biochem. Biophys. Res. Commun. 271, 492–498 (2000).
Yoshida, Y. et al. Human mitochondrial transcription factor A binds preferentially to oxidatively damaged DNA. Biochem. Biophys. Res. Commun. 295, 945–951 (2002).
Canugovi, C. et al. The mitochondrial transcription factor A functions in mitochondrial base excision repair. DNA Repair (Amst.) 9, 1080–1089 (2010).
Falkenberg, M. et al. Mitochondrial transcription factors B1 and B2 activate transcription of human mtDNA. Nat. Genet. 31, 289–294 (2002).
Sologub, M., Litonin, D., Anikin, M., Mustaev, A. & Temiakov, D. TFB2 is a transient component of the catalytic site of the human mitochondrial RNA polymerase. Cell 139, 934–944 (2009).
Cotney, J. & Shadel, G.S. Evidence for an early gene duplication event in the evolution of the mitochondrial transcription factor B family and maintenance of rRNA methyltransferase activity in human mtTFB1 and mtTFB2. J. Mol. Evol. 63, 707–717 (2006).
Seidel-Rogol, B.L., McCulloch, V. & Shadel, G.S. Human mitochondrial transcription factor B1 methylates ribosomal RNA at a conserved stem-loop. Nat. Genet. 33, 23–24 (2003).
Gaspari, M., Falkenberg, M., Larsson, N.G. & Gustafsson, C.M. The mitochondrial RNA polymerase contributes critically to promoter specificity in mammalian cells. EMBO J. 23, 4606–4614 (2004).
McCulloch, V. & Shadel, G.S. Human mitochondrial transcription factor B1 interacts with the C-terminal activation region of h-mtTFA and stimulates transcription independently of its RNA methyltransferase activity. Mol. Cell. Biol. 23, 5816–5824 (2003).
Garstka, H.L. et al. Import of mitochondrial transcription factor A (TFAM) into rat liver mitochondria stimulates transcription of mitochondrial DNA. Nucleic Acids Res. 31, 5039–5047 (2003).
Shutt, T.E., Lodeiro, M.F., Cotney, J., Cameron, C.E. & Shadel, G.S. Core human mitochondrial transcription apparatus is a regulated two-component system in vitro. Proc. Natl. Acad. Sci. USA 107, 12133–12138 (2010).
Maniura-Weber, K., Goffart, S., Garstka, H.L., Montoya, J. & Wiesner, R.J. Transient overexpression of mitochondrial transcription factor A (TFAM) is sufficient to stimulate mitochondrial DNA transcription, but not sufficient to increase mtDNA copy number in cultured cells. Nucleic Acids Res. 32, 6015–6027 (2004).
Pohjoismäki, J.L. et al. Alterations to the expression level of mitochondrial transcription factor A, TFAM, modify the mode of mitochondrial DNA replication in cultured human cells. Nucleic Acids Res. 34, 5815–5828 (2006).
Dairaghi, D.J., Shadel, G.S. & Clayton, D.A. Addition of a 29 residue carboxyl-terminal tail converts a simple HMG box-containing protein into a transcriptional activator. J. Mol. Biol. 249, 11–28 (1995).
Gangelhoff, T.A., Mungalachetty, P.S., Nix, J.C. & Churchill, M.E. Structural analysis and DNA binding of the HMG domains of the human mitochondrial transcription factor A. Nucleic Acids Res. 37, 3153–3164 (2009).
Masse, J.E. et al. The S. cerevisiae architectural HMGB protein NHP6A complexed with DNA: DNA and protein conformational changes upon binding. J. Mol. Biol. 323, 263–284 (2002).
Ohndorf, U.M., Rould, M.A., He, Q., Pabo, C.O. & Lippard, S.J. Basis for recognition of cisplatin-modified DNA by high-mobility-group proteins. Nature 399, 708–712 (1999).
Wong, T.S. et al. Biophysical characterizations of human mitochondrial transcription factor A and its binding to tumor suppressor p53. Nucleic Acids Res. 37, 6765–6783 (2009).
Hokari, M. et al. Overexpression of mitochondrial transcription factor A (TFAM) ameliorates delayed neuronal death due to transient forebrain ischemia in mice. Neuropathology 30, 401–407 (2010).
Gauthier, B.R. et al. PDX1 deficiency causes mitochondrial dysfunction and defective insulin secretion through TFAM suppression. Cell Metab. 10, 110–118 (2009).
Weinberg, F. et al. Mitochondrial metabolism and ROS generation are essential for Kras-mediated tumorigenicity. Proc. Natl. Acad. Sci. USA 107, 8788–8793 (2010).
Guo, J. et al. Frequent truncating mutation of TFAM induces mitochondrial DNA depletion and apoptotic resistance in microsatellite-unstable colorectal cancer. Cancer Res. 71, 2978–2987 (2011).
Stott, K., Tang, G.S., Lee, K.B. & Thomas, J.O. Structure of a complex of tandem HMG boxes and DNA. J. Mol. Biol. 360, 90–104 (2006).
Coll, M., Frederick, C.A., Wang, A.H. & Rich, A. A bifurcated hydrogen-bonded conformation in the d(A.T) base pairs of the DNA dodecamer d(CGCAAATTTGCG) and its complex with distamycin. Proc. Natl. Acad. Sci. USA 84, 8385–8389 (1987).
Williams, D.C. Jr., Cai, M. & Clore, G.M. Molecular basis for synergistic transcriptional activation by Oct1 and Sox2 revealed from the solution structure of the 42-kDa Oct1.Sox2.Hoxb1-DNA ternary transcription factor complex. J. Biol. Chem. 279, 1449–1457 (2004).
Klass, J. et al. The role of intercalating residues in chromosomal high-mobility-group protein DNA binding, bending and specificity. Nucleic Acids Res. 31, 2852–2864 (2003).
Love, J.J. et al. Structural basis for DNA bending by the architectural transcription factor LEF-1. Nature 376, 791–795 (1995).
Murphy, E.C., Zhurkin, V.B., Louis, J.M., Cornilescu, G. & Clore, G.M. Structural basis for SRY-dependent 46-X,Y sex reversal: modulation of DNA bending by a naturally occurring point mutation. J. Mol. Biol. 312, 481–499 (2001).
Sumitani, M., Kasashima, K., Matsugi, J. & Endo, H. Biochemical properties of Caenorhabditis elegans HMG-5, a regulator of mitochondrial DNA. J. Biochem. 149, 581–589 (2011).
Bernadó, P. Effect of interdomain dynamics on the structure determination of modular proteins by small-angle scattering. Eur. Biophys. J. 39, 769–780 (2010).
Sugimura, S. & Crothers, D.M. Stepwise binding and bending of DNA by Escherichia coli integration host factor. Proc. Natl. Acad. Sci. USA 103, 18510–18514 (2006).
Ohgaki, K. et al. The C-terminal tail of mitochondrial transcription factor a markedly strengthens its general binding to DNA. J. Biochem. 141, 201–211 (2007).
Gomis-Rüth, F.X. & Coll, M. Cut and move: protein machinery for DNA processing in bacterial conjugation. Curr. Opin. Struct. Biol. 16, 744–752 (2006).
Hyvärinen, A.K., Pohjoismaki, J.L., Holt, I.J. & Jacobs, H.T. Overexpression of MTERFD1 or MTERFD3 impairs the completion of mitochondrial DNA replication. Mol. Biol. Rep. 38, 1321–1328 (2011).
Konarev, P.V., Volkov, V.V., Sokolova, A.V., Koch, M.H.J. & Svergun, D.I. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 36, 1277–1282 (2003).
Bernadó, P., Mylonas, E., Petoukhov, M.V., Blackledge, M. & Svergun, D.I. Structural characterization of flexible proteins using small-angle X-ray scattering. J. Am. Chem. Soc. 129, 5656–5664 (2007).
Svergun, D.I., Barberato, C. & Koch, M.H.J. CRYSOL—a program to evaluate X-ray solution scattering of biological macromolecules from atomic coordinates. J. Appl. Crystallogr. 28, 768–773 (1995).
Kabsch, W. (ed.). Chapter 25.2.9: XDS 730–734 (Kluwer Academic Publishers (for The International Union of Crystallography), 2001).
CCP4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 50, 760–763 (1994).
Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 64, 112–122 (2008).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Lu, X.J. & Olson, W.K. 3DNA: a versatile, integrated software system for the analysis, rebuilding and visualization of three-dimensional nucleic-acid structures. Nat. Protoc. 3, 1213–1227 (2008).
Gouet, P., Courcelle, E., Stuart, D.I. & Metoz, F. ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15, 305–308 (1999).
Acknowledgements
We thank C. Silva and J. Colom for technical support. This study was supported by the Ministerio de Ciencia e Innovación (grants BFU2006-09593 to M.S., BFU2009-07134 to M.S., BFU2008-02372 to M.C., CSD2006-00023), Generalitat de Catalunya (SGR2009-1366 to M.S., SGR2009-1309 to M.C., SGR2009-1352 to P.B.), the European Union (FP7-HEALTH-2010-261460 to M.S., FP7-BioNMR-2010-261863 to P.B.), and Instituto de Salud Carlos III-FIS-PI 10/00662. The Centro de Investigación Biomédica en Red de Enfermedades Raras is an initiative of the Instituto de Salud Carlos III. A.R.-C., J.F.S., N.J.-M. and P.F.-M. hold or held fellowships from Consejo Superior de Investigaciones Científicas, MICINN and Cusanswerk-Bischöfliche Studienförderung. H.T.J. is supported by Academy of Finland, Tampere University Hospital Medical Research Fund and Sigrid Juselius Foundation. We also thank the European Molecular Biology Laboratory (EMBL)-Grenoble and EMBL-Hamburg Outstations, the European Synchrotron Radiation Facility in Grenoble and the Automated Crystallography Platform (Barcelona Science Park) for their support.
Author information
Authors and Affiliations
Contributions
A.R.-C. and J.F.S. contributed to cloning, protein production and crystallization; A.R.-C., N.J.-M. P.F.-M. and P.B. conducted the SAXS studies; A.R.-C. and M.S. contributed to X-ray structure solution; A.R.-C. and M.S. contributed to figure preparation. Together with the rest of authors, M.C., J.M. and H.T.J. participated in manuscript writing, provision of materials and infrastructure, and discussion. M.S. designed and supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–3 (PDF 1728 kb)
Rights and permissions
About this article
Cite this article
Rubio-Cosials, A., Sydow, J., Jiménez-Menéndez, N. et al. Human mitochondrial transcription factor A induces a U-turn structure in the light strand promoter. Nat Struct Mol Biol 18, 1281–1289 (2011). https://doi.org/10.1038/nsmb.2160
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nsmb.2160
This article is cited by
-
Mechanisms and regulation of human mitochondrial transcription
Nature Reviews Molecular Cell Biology (2024)
-
Expression of mitochondrial transcription factor A in granulosa cells: implications for oocyte maturation and in vitro fertilization outcomes
Journal of Assisted Reproduction and Genetics (2024)
-
A recessive variant in TFAM causes mtDNA depletion associated with primary ovarian insufficiency, seizures, intellectual disability and hearing loss
Human Genetics (2021)
-
New protein-DNA complexes in archaea: a small monomeric protein induces a sharp V-turn DNA structure
Scientific Reports (2019)
-
Structural basis of mitochondrial transcription
Nature Structural & Molecular Biology (2018)