Key Points
-
To be effective in enabling growth-cone motility, actin filaments must be organized into higher-order assemblies with distinct architectures (actin superstructures).
-
Actin superstructures are self-organizing. The cell-free Listeria monocytogenes comet-tail reconstitution assay demonstrated that a propulsive and dynamic actin superstructure can be self-organized by an ensemble of actin-binding proteins. Although they are classically defined only by their architectural appearance, actin superstructures can now be more subtly distinguished by their recruitment of specific ensembles of actin-binding proteins.
-
Actin superstructure organization effectively occurs in a two-step process. As actin superstructures are self-organizing, the limiting step is actin-filament assembly itself. Not all of the proteins that are involved in generating or maintaining actin superstructures have been well characterized in growth cones.
-
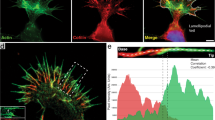
Leading-edge actin superstructures are organised into stratified layers. Because actin subunits incorporate into filaments at the leading-edge membrane, migrate away from the membrane at similar rates, and undergo ATP hydrolysis and Pi release on an average time scale, actin superstructures can be divided into layers or strata based on the enrichment of specific actin–nucleotide complexes or the enriched binding of certain actin-binding proteins.
-
A single actin-binding protein alone can support either an attractive or a repulsive turn. In Xenopus laevis spinal neuronal growth cones, a gradient of bone morphogenetic protein 7 (BMP7) can cause either an attractive or a repulsive turn through the bidirectional phospho-regulation of the actin-binding protein cofilin. Attractive turning requires the activation of a kinase pathway, whereas repulsive turning requires the activation of a Ca2+-dependent phosphatase pathway. Thus, actin-binding proteins can function as effective 'signal integrators' of signalling pathways.
-
Distinct but spatially overlapping actin superstructures exist. With the advent of fluorescent speckle microscopy, dynamic characteristics of actin superstructures can be measured and used to identify distinct but spatially overlapping superstructures. In migrating non-neuronal cells, the generation and maintenance of distinct but overlapping superstructures requires the tropomyosin proteins, and although distinct but overlapping superstructures have not been identified in growth cones, all of the required components are present.
-
Actin superstructures have not been described in dystrophic growth cones. In response to axotomy, the tips of CNS axons undergo a morphological change to become dystrophic growth cones. Although actin has been suggested to be a potential target for regenerative therapy, it is not even clear whether actin superstructures are reassembled in dystrophic growth cones.
-
New approaches, ideas and technologies should be considered in the study of actin-based growth-cone motility. In recognizing that actin superstructure organization occurs in a two-step process, two outstanding issues become clear. First, the mechanisms that control actin nucleation and growth require further characterization in growth cones. Second, the proteome of actin-binding proteins that underlie unique actin superstructures should be determined for growth cones. Emerging micro- and nano-technologies should greatly facilitate future studies.
Abstract
Higher-order actin-based networks (actin superstructures) are important for growth-cone motility and guidance. Principles for generating, organizing and remodelling actin superstructures have emerged from recent findings in cell-free systems, non-neuronal cells and growth cones. This Review examines how actin superstructures are initiated de novo at the leading-edge membrane and how the spontaneous organization of actin superstructures is driven by ensembles of actin-binding proteins. How the regulation of actin-binding proteins can affect growth-cone turning and axonal regeneration is also discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Marsh, L. & Letourneau, P. C. Growth of neurites without filopodial or lamellipodial activity in the presence of cytochalasin B. J. Cell Biol. 99, 2041–2047 (1984). This was the first study to demonstrate that actin superstructures are dispensable for neurite elongation.
Letourneau, P. C., Shattuck, T. A. & Ressler, A. H. “Pull” and “push” in neurite elongation: observations on the effects of different concentrations of cytochalasin B and taxol. Cell Motil. Cytoskeleton 8, 193–209 (1987).
Bradke, F. & Dotti, C. G. The role of local actin instability in axon formation. Science 283, 1931–1934 (1999).
Bentley, D. & Toroian-Raymond, A. Disoriented pathfinding by pioneer neuron growth cones deprived of filopodia by cytochalasin treatment. Nature 323, 712–715 (1986). Although actin superstructures are not required for neurite elongation, this paper was the first to demonstrate a role for actin superstructures in growth-cone pathfinding in vivo.
Zheng, J. Q., Wan, J. J. & Poo, M. M. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J. Neurosci. 16, 1140–1149 (1996).
Zhou, F. Q., Waterman-Storer, C. M. & Cohan, C. S. Focal loss of actin bundles causes microtubule redistribution and growth cone turning. J. Cell Biol. 157, 839–849 (2002).
Rajnicek, A. M., Foubister, L. E. & McCaig, C. D. Growth cone steering by a physiological electric field requires dynamic microtubules, microfilaments and Rac-mediated filopodial asymmetry. J. Cell Sci. 119, 1736–1745 (2006).
Gallo, G. & Letourneau, P. C. Regulation of growth cone actin filaments by guidance cues. J. Neurobiol. 58, 92–102 (2004).
Rodriguez, O. C. et al. Conserved microtubule–actin interactions in cell movement and morphogenesis. Nature Cell Biol. 5, 599–609 (2003).
Condeelis, J. Life at the leading edge: the formation of cell protrusions. Annu. Rev. Cell Biol. 9, 411–444 (1993).
Loisel, T. P., Boujemaa, R., Pantaloni, D. & Carlier, M. F. Reconstitution of actin-based motility of Listeria and Shigella using pure proteins. Nature 401, 542–543 (1999).
Cameron, L. A., Footer, M. J., van Oudenaarden, A. & Theriot, J. A. Motility of ActA protein-coated microspheres driven by actin polymerization. Proc. Natl Acad. Sci. USA 96, 4908–4913 (1999). This paper, together with reference 11, reported the complete reconstitution of L. monocytogenes actin-based comet-tail motility using a cell-free system, demonstrating that a force-producing actin-based machine could be assembled without external signalling or motor proteins.
Mitchison, T. J. Self-organization of polymer-motor complexes in the cytoskeleton. Philos. Trans. R. Soc. Lond. B Biol. Sci. 336, 99–106 (1992).
Pantaloni, D., Le Clainche, C. & Carlier, M. F. Mechanism of actin-based motility. Science 292, 336–345 (2001).
Sampath, P. & Pollard, T. D. Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry 30, 1973–1980 (1991).
Forscher, P. & Smith, S. J. Actions of cytochalasins on the organization of actin filaments and microtubules in a neuronal growth cone. J. Cell Biol. 107, 1505–1516 (1988). By reversibly blocking actin assembly, this study demonstrated that growth-cone actin superstructures are assembled de novo at the leading edge.
Sept, D. & McCammon, J. A. Thermodynamics and kinetics of actin filament nucleation. Biophys. J. 81, 667–674 (2001).
Mullins, R. D., Heuser, J. A. & Pollard, T. D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl Acad. Sci. USA 95, 6181–6186 (1998).
Pruyne, D. et al. Role of formins in actin assembly: nucleation and barbed-end association. Science 297, 612–615 (2002).
Quinlan, M. E., Heuser, J. E., Kerkhoff, E. & Mullins, R. D. Drosophila Spire is an actin nucleation factor. Nature 433, 382–388 (2005).
Andrianantoandro, E. & Pollard, T. D. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24, 13–23 (2006).
Kerkhoff, E. Cellular functions of the Spir actin-nucleation factors. Trends Cell Biol. 16, 477–483 (2006).
Takenawa, T. & Suetsugu, S. The WASP–WAVE protein network: connecting the membrane to the cytoskeleton. Nature Rev. Mol. Cell Biol. 8, 37–48 (2007).
Faix, J. & Grosse, R. Staying in shape with formins. Dev. Cell 10, 693–706 (2006).
Goley, E. D. & Welch, M. D. The Arp2/3 complex: an actin nucleator comes of age. Nature Rev. Mol. Cell Biol. 7, 713–726 (2006).
Bamburg, J. R. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 15, 185–230 (1999).
Higgs, H. N. & Pollard, T. D. Activation by Cdc42 and PIP2 of Wiskott-Aldrich syndrome protein (WASp) stimulates actin nucleation by Arp2/3 complex. J. Cell Biol. 150, 1311–1320 (2000).
Bassell, G. J. et al. Sorting of β-actin mRNA and protein to neurites and growth cones in culture. J. Neurosci. 18, 251–265 (1998).
Leung, K. M. et al. Asymmetrical β-actin mRNA translation in growth cones mediates attractive turning to netrin-1. Nature Neurosci. 9, 1247–1256 (2006).
Yao, J., Sasaki, Y., Wen, Z., Bassell, G. J. & Zheng, J. Q. An essential role for β-actin mRNA localization and translation in Ca2+-dependent growth cone guidance. Nature Neurosci. 9, 1265–1273 (2006).
Lin, A. C. & Holt, C. E. Local translation and directional steering in axons. EMBO J. 26, 3729–3736 (2007).
Paunola, E., Mattila, P. K. & Lappalainen, P. WH2 domain: a small, versatile adapter for actin monomers. FEBS Lett. 513, 92–97 (2002).
Carlier, M. F. & Pantaloni, D. Control of actin dynamics in cell motility. J. Mol. Biol. 269, 459–467 (1997).
Paglini, G., Kunda, P., Quiroga, S., Kosik, K. & Caceres, A. Suppression of radixin and moesin alters growth cone morphology, motility, and process formation in primary cultured neurons. J. Cell Biol. 143, 443–455 (1998).
Castelo, L. & Jay, D. G. Radixin is involved in lamellipodial stability during nerve growth cone motility. Mol. Biol. Cell 10, 1511–1520 (1999).
Polesello, C. & Payre, F. Small is beautiful: what flies tell us about ERM protein function in development. Trends Cell Biol. 14, 294–302 (2004).
Kovar, D. R., Harris, E. S., Mahaffy, R., Higgs, H. N. & Pollard, T. D. Control of the assembly of ATP- and ADP-actin by formins and profilin. Cell 124, 423–435 (2006).
Co, C., Wong, D. T., Gierke, S., Chang, V. & Taunton, J. Mechanism of actin network attachment to moving membranes: barbed end capture by N-WASP WH2 domains. Cell 128, 901–913 (2007).
Trichet, L., Campas, O., Sykes, C. & Plastino, J. VASP governs actin dynamics by modulating filament anchoring. Biophys. J. 92, 1081–1089 (2007).
Wolfram, S. Cellular automata. Los Alamos Science, 9, Fall (1983).
Carlier, M. F. Actin polymerization and ATP hydrolysis. Adv. Biophys. 26, 51–73 (1990).
Galkin, V. E. et al. ADF/cofilin use an intrinsic mode of F-actin instability to disrupt actin filaments. J. Cell Biol. 163, 1057–1066 (2003).
Cai, L., Makhov, A. M. & Bear, J. E. F-actin binding is essential for coronin 1B function in vivo. J. Cell Sci. 120, 1179–1790 (2007).
Weed, S. A. et al. Cortactin localization to sites of actin assembly in lamellipodia requires interactions with F-actin and the Arp2/3 complex. J. Cell Biol. 151, 29–40 (2000).
Loureiro, J. J. et al. Critical roles of phosphorylation and actin binding motifs, but not the central proline-rich region, for Ena/vasodilator-stimulated phosphoprotein (VASP) function during cell migration. Mol. Biol. Cell 13, 2533–2546 (2002).
Svitkina, T. M. & Borisy, G. G. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J. Cell Biol. 145, 1009–1026 (1999).
Karakozova, M. et al. Arginylation of β-actin regulates actin cytoskeleton and cell motility. Science 313, 192–196 (2006).
Cohan, C. S., Welnhofer, E. A., Zhao, L., Matsumura, F. & Yamashiro, S. Role of the actin bundling protein fascin in growth cone morphogenesis: localization in filopodia and lamellipodia. Cell Motil. Cytoskeleton 48, 109–120 (2001).
McGough, A., Pope, B., Chiu, W. & Weeds, A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J. Cell Biol. 138, 771–781 (1997).
Galkin, V. E., Orlova, A., Lukoyanova, N., Wriggers, W. & Egelman, E. H. Actin depolymerizing factor stabilizes an existing state of F-actin and can change the tilt of F-actin subunits. J. Cell Biol. 153, 75–86 (2001).
Friederich, E. et al. Targeting of Listeria monocytogenes ActA protein to the plasma membrane as a tool to dissect both actin-based cell morphogenesis and ActA function. EMBO J. 14, 2731–2744 (1995).
Gassama-Diagne, A. et al. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nature Cell Biol. 8, 963–970 (2006).
Moon, M. S. & Gomez, T. M. Adjacent pioneer commissural interneuron growth cones switch from contact avoidance to axon fasciculation after midline crossing. Dev. Biol. 288, 474–486 (2005).
Gallo, G., Yee, H. F. & Letourneau, P. C. Actin turnover is required to prevent axon retraction by endogenous actomyosin contractility. J. Cell Biol. 158, 1219–1228 (2002).
Medeiros, N. A., Burnette, D. T. & Forscher, P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nature Cell Biol. 8, 215–226 (2006).
Carlier, M. F. et al. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J. Cell Biol. 136, 1307–1322 (1997).
Mallavarapu, A. & Mitchison, T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J. Cell Biol. 146, 1097–1106 (1999).
Turney, S. G. & Bridgman, P. C. Laminin stimulates and guides axonal outgrowth via growth cone myosin II activity. Nature Neurosci. 8, 717–719 (2005).
Wen, Z. et al. BMP gradients steer nerve growth cones by a balancing act of LIM kinase and Slingshot phosphatase on ADF/cofilin. J. Cell Biol. 178, 107–119 (2007). The results in this paper stand alone in demonstrating that the bidirectional regulation of a single type of actin-binding protein can mediate both attractive and repulsive turning in response to the same extracellular guidance cue.
Aizawa, H. et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nature Neurosci. 4, 367–373 (2001).
Gallo, G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A-induced axonal retraction. J. Cell Sci. 119, 3413–3423 (2006).
Song, H. J. & Poo, M. M. Signal transduction underlying growth cone guidance by diffusible factors. Curr. Opin. Neurobiol. 9, 355–363 (1999).
Henley, J. R., Huang, K. H., Wang, D. & Poo, M. M. Calcium mediates bidirectional growth cone turning induced by myelin-associated glycoprotein. Neuron 44, 909–916 (2004).
Falk, J. et al. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron 48, 63–75 (2005).
Agnew, B. J., Minamide, L. S. & Bamburg, J. R. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J. Biol. Chem. 270, 17582–17587 (1995).
Moriyama, K., Iida, K. & Yahara, I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells 1, 73–86 (1996).
Arber, S. et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 398, 805–809 (1998).
Yang, N. et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin organization. Nature 393, 809–812 (1998).
Niwa, R., Nagata-Ohashi, K., Takeichi, M., Mizuno, K. & Uemura, T. Control of actin reorganization by slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell 108, 233–246 (2002).
Soosairajah, J. et al. Interplay between components of a novel LIM kinase–slingshot phosphatase complex regulates cofilin. EMBO J. 24, 473–86 (2005).
Foletta, V. C. et al. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J. Cell Biol. 162, 1089–1098 (2003).
Greka, A., Navarro, B., Oancea, E., Dugga, A. & Clapham, D. E. TRPC5 is a regulator of hippocampal neurite length and growth cone morphology. Nature Neurosci. 6, 837–845 (2003).
Wang, G. X. & Poo, M. M. Requirement of TRPC channels in netrin-1-induced chemotropic turning of nerve growth cones. Nature 434, 898–904 (2005).
Li, Y. et al. Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434, 894–898 (2005).
Shim, S. et al. XTRPC1-dependent chemotropic guidance of neuronal growth cones. Nature Neurosci. 8, 730–735 (2005).
Waterman-Storer, C. M., Desai, A., Bulinski, J. C. & Salmon, E. D. Fluorescent speckle microscopy, a method to visualize the dynamics of protein assemblies in living cells. Curr. Biol. 8, 1227–1230 (1998). This paper describes a method for visualizing actin in both microtubules and actin-filament networks as single diffraction-limited molecules from which dynamic parameters of subunit behaviour can be measured.
Ponti, A., Machacek, M., Gupton, S. L., Waterman-Storer, C. M. & Danuser, G. Two distinct actin networks drive the protrusion of migrating cells. Science 305, 1782–1786 (2004). This study used FSM to identify two populations of actin speckles that correspond to actin subunits from lamellipodia and lamellae.
Gupton, S. L. et al. Cell migration without a lamellipodium: translation of actin dynamics into cell movement mediated by tropomyosin. J. Cell Biol. 168, 619–631 (2005).
Iwasa, J. H. & Mullins, R. D. Spatial and temporal relationships between actin-filament nucleation, capping, and disassembly. Curr. Biol. 17, 395–406 (2007).
Delorme, V. et al. Cofilin activity downstream of pak1 regulates cell protrusion efficiency by organizing lamellipodium and lamella actin networks. Dev. Cell 13, 646–662 (2007).
Schaefer, A. W., Kabir, N. & Forscher, P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J. Cell Biol. 158, 139–152 (2002).
Gunning, P. W., Schevzov, G., Kee, A. J. & Hardeman, E. C. Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends Cell Biol. 15, 333–341 (2005).
Schevzov, G. et al. Tropomyosin localization reveals distinct populations of microfilaments in neurites and growth cones. Mol. Cell. Neurosci. 8, 439–454 (1997).
Bryce, N. S. et al. Specifications of actin filament function and molecular composition by tropomyosin isoforms. Mol. Biol. Cell 14, 1002–1016 (2003). This study was the first to demonstrate that both competitive and synergistic interactions between different tropomyosin isoforms and ADF/cofilin regulate binding to different actin-filament populations.
Bernstein, B. W. & Bamburg, J. R. Tropomyosin binding to F-actin protects the F-actin from disassembly by brain actin depolymerizing factor (ADF). Cell Motil. 2, 1–8 (1982).
Nishida, E., Muneyuki, E., Maekawa, S., Ohta, Y. & Sakai, H. An actin-depolymerizing protein (destrin) from porcine kidney. Its action on F-actin containing or lacking tropomyosin. Biochemistry 24, 6624–6630 (1985).
DesMarais, V., Ichetovkin, I., Condeelis, J. & Hitchcock-DeGregori, S. E. Spatial regulation of actin dynamics: a tropomyosin-free, actin-rich compartment at the leading edge. J. Cell Biol. 115, 4649–4660 (2002).
Schevzov, G. et al. Specific features of neuronal size and shape are regulated by tropomyosin isoforms. Mol. Biol. Cell 16, 3425–3437 (2005).
Yiu, G. & He, Z. Glial inhibition of CNS axon regeneration. Nature Rev. Neurosci. 7, 617–627 (2006).
David, S. & Aguayo, A. J. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science 214, 931–933 (1981).
Kuhn, T. B., Brown, M. D., Wilcox, C. L., Raper, J. A. & Bamburg, J. R. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of rac1. J. Neurosci. 19, 1965–1975 (1999).
Yuan, X. B. et al. Signalling and crosstalk of Rho GTPases in mediating axon guidance. Nature Cell Biol. 5, 38–45 (2003).
Niederost, B., Oertle, T., Fritsche, J., McKinney, R. A. & Bandtlow, C. E. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J. Neurosci. 22, 10368–10376 (2002).
Hsieh, S. H., Ferraro, G. B. & Fournier, A. E. Myelin-associated inhibitors regulate cofilin phosphorylation and neuronal inhibition through LIM kinase and Slingshot phosphatase. J. Neurosci. 26, 1006–1015 (2006).
Tom, V. J., Steinmetz, M. P., Miller, J. H., Doller, C. M. & Silver, J. Studies on the development and behavior of the dystrophic growth cone, the hallmark of regeneration failure, in an in vitro model of the glial scar and after spinal cord injury. J. Neurosci. 24, 6531–6539 (2004).
Weibel, D. B., Garstecki, P. & Whitesides, G. M. Combining microscience and neurobiology. Curr. Opin. Neurobiol. 15, 560–567 (2005).
Gross, P. G., Kartalov, E. P., Scherer, A. & Weiner, L. P. Applications of microfluidics for neuronal studies. J. Neurol. Sci. 252, 135–143 (2007).
Burg, T. P. et al. Weighing of biomolecules, single cells and single nanoparticles in fluid. Nature 446, 1066–1069 (2007).
Wegner, A. Head to tail polymerization of actin. J. Mol. Biol. 108, 139–150 (1976). This was the first report of actin treadmilling.
Hill, T. L. & Kirschner, M. W. Subunit treadmilling of microtubules or actin in the presence of cellular barriers: possible conversion of chemical free energy into mechanical work. Proc. Natl Acad. Sci. USA 79, 490–494 (1982). This seminal paper provided the theoretical basis for the concept that cytoskeleton proteins can function as 'molecular machines' owing to their ability to treadmill — a concept that has proven integral to current models of actin-based motility.
Isambert, H. et al. Flexibility of actin filaments derived from thermal fluctuations. Effect of bound nucleotide, phalloidin, and muscle regulatory proteins. J. Biol. Chem. 270, 11437–11444 (1995).
Sato, M., Schwarz, W. H. & Pollard, T. D. Dependence of the mechanical properties of actin/α-actinin gels on deformation rate. Nature 325, 828–830 (1987).
Gardel, M. L. et al. Elastic behavior of cross-linked and bundled actin networks. Science 304, 1301–1305 (2004).
Wachsstock, D. H., Schwarz, W. H. & Pollard, T. D. Cross-linker dynamics determine the mechanical properties of actin gels. Biophys. J. 66, 801–809 (1994).
Claessens, M. M., Bathe, M., Frey, E. & Bausch, A. R. Actin-binding proteins sensitively mediate F-actin bundle stiffness. Nature Mater. 5, 748–753 (2006).
Wachsstock, D. H., Schwartz, W. H. & Pollard, T. D. Affinity of alpha-actinin for actin determines the structure and mechanical properties of actin filament gels. Biophys. J. 65, 205–214 (1993).
Strasser, G. A., Rahim, N. A., VanderWall, K. E., Gertler, F. B. & Lanier, L. M. Arp2/3 is a negative regulator of growth cone translocation. Neuron 43, 81–94 (2004).
Goldberg, D. J., Foley, M. S., Tang, D. & Grabham, P. W. Recruitment of the Arp2/3 complex and Mena for the stimulation of actin polymerization in growth cones by nerve growth factor. J. Neurosci. Res. 60, 458–467 (2000).
Mongiu, A. K., Weitzke, E. L., Chaga, O. Y. & Borisy, G. G. Kinetic-structural analysis of neuronal growth cone veil motility. J. Cell Sci. 120, 1113–1125 (2007).
Romero, S. et al. Formin is a processive motor that requires profilin to accelerate actin assembly and associated ATP hydrolysis. Cell 119, 419–429 (2004).
Arakawa, Y. et al. Control of axon elongation via an SDF-1α/Rho/mDia pathway in cultured cerebellar granule neurons. J. Cell Biol. 161, 381–391 (2003).
Mouneimne, G. et al. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J. Cell Biol. 166, 697–708 (2004).
Song, X. et al. Initiation of cofilin activity in response to EGF is uncoupled from cofilin phosphorylation and dephosphorylation in carcinoma cells. J. Cell Sci. 119, 2871–2881 (2006).
Acknowledgements
The authors would like to thank K. Hite and E. Peterson for valuable discussions and critical reading of the manuscript, and the National Science Foundation grant DGE0234615 (C.W.P.) and National Institutes of Health grants NS48660 (K.C.F.) and NS40371 (J.R.B.) for financial support.
Author information
Authors and Affiliations
Corresponding author
Glossary
- Barbed end
-
The end of an actin filament that polymerizes faster when the actin-monomer concentration is high and that also polymerizes at steady-state.
- Pointed end
-
The end of an actin filament that polymerizes slower when the actin-monomer concentration is high and that depolymerizes at steady state.
- Lamellipodium
-
A specific leading-edge cellular protrusion that is characterized by branched actin filaments, the absence of high-molecular-weight tropomyosins and the presence of cofilin. It is localized within 1–2 μm of the leading edge. Actin speckles in lamellipodia undergo fast retrograde flow and are short-lived. Mesh-like actin-based protrusions in neuronal growth cones are sometimes referred to as lamellipodia.
- Actin speckle
-
Fluorescently-tagged actin monomers that appear as single diffraction-limited molecules when expressed in cells at a low concentration.
- Lamellum
-
A leading-edge cellular protrusion that is characterized by the presence of less-branched actin filaments than those in lamellipodia and the recruitment of high-molecular-weight (HMW) tropomyosins and myosin. It is localized within 2–5 microns of the leading edge. Actin speckles in the lamellae undergo slower retrograde flow and are longer-lived than those in lamellipodia.
Rights and permissions
About this article
Cite this article
Pak, C., Flynn, K. & Bamburg, J. Actin-binding proteins take the reins in growth cones. Nat Rev Neurosci 9, 136–147 (2008). https://doi.org/10.1038/nrn2236
Issue Date:
DOI: https://doi.org/10.1038/nrn2236
This article is cited by
-
Advances in Understanding the Molecular Mechanisms of Neuronal Polarity
Molecular Neurobiology (2023)
-
Fascin-1 Contributes to Neuropathic Pain by Promoting Inflammation in Rat Spinal Cord
Neurochemical Research (2018)
-
SYD-1 Promotes Multiple Developmental Steps Leading to Neuronal Connectivity
Molecular Neurobiology (2016)
-
The role of myosin-II in force generation of DRG filopodia and lamellipodia
Scientific Reports (2015)
-
Analysis of nuclear actin by overexpression of wild-type and actin mutant proteins
Histochemistry and Cell Biology (2014)