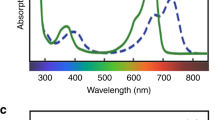
Key Points
-
The phytochromes comprise a small family of sensory photoreceptors (with five members in Arabidopsis, designated phyA–phyE), which monitor informational light signals in the environment and modulate appropriate growth and development responses through induced changes in gene expression.
-
Individual family members have different, albeit frequently partially overlapping, photosensory and/or physiological functional roles in regulating plant responses to light.
-
Five principal developments in recent years have substantially influenced current concepts of the cellular, molecular and biochemical mechanisms by which the phytochromes perceive and transduce light signals to photoresponsive nuclear genes:
— light-induced nuclear translocation of phytochrome molecules;
— direct interaction of phytochrome molecules with a DNA-bound transcription factor;
— transcription-factor genes are early targets of phytochrome signalling;
— phytochrome-associated protein kinase activity;
— post-translational regulation of signalling through targeted degradation of a key transcriptional regulator.
-
Evidence so far indicates that the phytochromes might use at least two pathways to signal to photoresponsive genes: a primary, early-response pathway, which involves transcriptional regulation by direct targeting of light signals to responsive promoters; and a secondary, longer-term pathway, which involves abrogation of targeted proteolytic degradation of a key transcription factor.
-
Future research efforts can be expected to exploit the unprecedented power that is offered by the combination of mutants and microarrays for the dissection of photosensory signalling and transcriptional networks.
Abstract
Light is life for plants. To continuously assess and adapt to fluctuations in the quality and quantity of this essential commodity, plants deploy sensory photoreceptors, including the phytochromes. Having captured an incoming photon, the activated phytochrome molecule must relay this information to nuclear genes that are poised to respond by directing appropriate adjustments in growth and development. Defining the intricate intracellular signalling networks through which this sensory information is transduced is an area of intense research activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kendrick, R. E. & Kronenberg, G. H. M. Photomorphogenesis in Plants 2nd edn (Kluwer Academic, Dordrecht, The Netherlands, 1994).
Cashmore, A. R., Jarillo, J. A., Wu, Y. J. & Liu, D. Cryptochromes: blue light receptors for plants and animals. Science 284, 760–765 (1999).
Briggs, W. R. et al. The phototropin family of photoreceptors. Plant Cell 13, 993–997 (2001).
Neff, M. M., Fankhauser, C. & Chory, J. Light: an indicator of time and place. Genes Dev. 14, 257–271 (2000).
Smith, H. Phytochromes and light signal perception by plants — an emerging synthesis. Nature 407, 585–591 (2000). This review provides a succinct, up-to-date overview of the phytochrome system.
Fankhauser, C. The phytochromes, a family of red/far-red absorbing photoreceptors. J. Biol. Chem. 276, 11453–11456 (2001).
Deng, X.-W. & Quail, P. H. Signalling in light-controlled development. Semin. Cell Dev. Biol. 10, 121–129 (1999).
Chory, J. & Wu, D. Y. Weaving the complex web of signal transduction. Plant Physiol. 125, 77–80 (2001).
Quail, P. H. An emerging molecular map of the phytochromes. Plant Cell Environ. 20, 657–665 (1997).
Mathews, S., Lavin, M. & Sharrock, R. A. Evolution of the phytochrome gene family and its utility for phylogenetic analyses of angiosperms. Anal. Missouri Bot. Gard. 82, 296–321 (1995).
Mathews, S. & Sharrock, R. A. Phytochrome gene diversity. Plant Cell Environ. 20, 666–671 (1997).
Schneider-Poetsch, H. A. W., Kolukisaoglu, Ü., Clapham, D. H., Hughes, J. & Lamparter, T. Non-angiosperm phytochromes and the evolution of vascular plants. Physiol. Plant. 102, 612–622 (1998).
Hughes, J. et al. A prokaryotic phytochrome. Nature 386, 663–664 (1997).
Yeh, K.-C., Wu, S.-H., Murphy, J. T. & Lagarias, J. C. A cyanobacterial phytochrome two-component light sensory system. Science 277, 1505–1508 (1997). This paper documents that a cyanobacterial protein, predicted from whole genome sequencing (Ref. 84 ) to have an amino-terminal phytochrome photosensory domain and a carboxyl-terminal histidine-kinase domain, does indeed function in vitro as a light-regulated sensor histidine kinase, which is capable of transphosphorylation of its presumptive cognate response regulator in a classical prokaryotic two-component fashion.
Bhoo, S.-H., Davis, S. J., Walker, J., Karniol, B. & Vierstra, R. D. Bacteriophytochromes are photochromic histidine kinases using a biliverdin chromophore. Nature 414, 776–778 (2001).
Vierstra, R. D. & Davis, S. J. Bacteriophytochromes: new tools for understanding phytochrome signal transduction. Semin. Cell Dev. Biol. 11, 511–521 (2000).
Wu, S. H. & Lagarias, J. C. Defining the bilin lyase domain: lessons from the extended phytochrome superfamily. Biochemistry 39, 13487–13495 (2000).
Whitelam, G. C. & Devlin, P. F. Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Environ. 20, 752–758 (1997).
Devlin, P. F., Patel, S. R. & Whitelam, G. C. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 10, 1479–1487 (1998).
Quail, P. H. The phytochrome family: dissection of functional roles and signalling pathways among family members. Phil. Trans. R. Soc. Lond. B 353, 1399–1403 (1998).
Quail, P. H. et al. Phytochromes: photosensory perception and signal transduction. Science 268, 675–680 (1995).
Fankhauser, C. & Chory, J. Light control of plant development. Annu. Rev. Cell Dev. Biol. 13, 203–229 (1997).
Hudson, M. E. The genetics of phytochrome signalling in Arabidopsis. Semin. Cell Dev. Biol. 11, 475–483 (2000).
Quail, P. H. Phytochrome interacting factors. Semin. Cell Dev. Biol. 11, 457–466 (2000).
Hudson, M., Ringli, C., Boylan, M. T. & Quail, P. H. The FAR1 locus encodes a novel nuclear protein specific to phytochrome A signalling. Genes Dev. 13, 2017–2027 (1999).
Fairchild, C. D., Schumaker, M. A. & Quail, P. H. HFR1 encodes an atypical bHLH protein that acts in phytochrome A signal transduction. Genes Dev. 14, 2377–2391 (2000).
Soh, M. S., Kim, Y. M., Han, S. J. & Song, P. S. REP1, a basic helix-loop-helix protein, is required for a branch pathway of phytochrome A signalling in Arabidopsis. Plant Cell 12, 2061–2073 (2000).
Spiegelman, J. I. et al. Cloning of the Arabidopsis RSF1 gene by using a mapping strategy based on high-density DNA arrays and denaturing high-performance liquid chromatography. Plant Cell 12, 2485–2498 (2000).
Hoecker, U., Tepperman, J. M. & Quail, P. H. SPA1: a WD-repeat protein specific to phytochrome A signal transduction. Science 284, 496–499 (1999).
Ballesteros, M. L. et al. LAF1, a MYB transcription activator for phytochrome A signalling. Genes Dev. 15, 2613–2615 (2001).
Dieterle, M., Zhou, Y. C., Schäfer, E., Funk, M. & Kretsch, T. EID1, an F-box protein involved in phytochrome A-specific light signalling. Genes Dev. 15, 939–944 (2001).
Ni, M., Tepperman, J. M. & Quail, P. H. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95, 657–667 (1998).
Huq, E., Tepperman, J. M. & Quail, P. H. GIGANTEA is a nuclear protein involved in phytochrome signalling in Arabidopsis. Proc. Natl Acad. Sci. USA 97, 9789–9794 (2000).
Liu, X. L., Covington, M. F., Fankhauser, C., Chory, J. & Wagner, D. R. Y. ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis phyB signal transduction pathway. Plant Cell 13, 1293–1304 (2001).
Pepper, A., Delaney, T., Washburn, T., Poole, D. & Chory, J. DET1, a negative regulator of light-mediated development and gene expression in Arabidopsis, encodes a novel nuclear-localized protein. Cell 78, 109–116 (1994).
Schwechheimer, C. & Deng, X. W. The COP/DET/FUS proteins — regulators of eukaryotic growth and development. Semin. Cell Dev. Biol. 11, 495–503 (2000).
Hardtke, C. S. & Deng, X. W. The cell biology of the COP/DET/FUS proteins. Regulating proteolysis in photomorphogenesis and beyond? Plant Physiol. 124, 1548–1557 (2000).
Quail, P. H. Phytochrome: a light-activated molecular switch that regulates plant gene expression. Annu. Rev. Genet. 25, 389–409 (1991).
Pratt, L. H. In Photomorphogenesis in Plants 2nd Edn (eds Kendrich, R. E. & Kronenburg, G. H. M.) 163–185 (Kluwer Academic, Dordrecht, The Netherlands, 1994).
Millar, A. J., McGrath, R. B. & Chua, N.-H. Phytochrome phototransduction pathways. Annu. Rev. Genet. 28, 325–349 (1994).
Schäfer, E., Kunkel, T. & Frohnmeyer, H. Signal transduction in the photocontrol of chalcone synthase gene expression. Plant Cell Environ. 20, 722–727 (1997).
Okamoto, H., Matsui, M. & Deng, X. W. Overexpression of the heterotrimeric G-protein α-subunit enhances phytochrome-mediated inhibition of hypocotyl elongation in Arabidopsis. Plant Cell 13, 1639–1651 (2001).
Guo, H., Mockler, T., Duong, H. & Lin, C. SUB1, an arabidopsis Ca2+-binding protein involved in cryptochrome and phytochrome coaction. Science 291, 487–490 (2001).
Arabidopsis Genome Initiative. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815 (2000).
Halliday, K. J., Hudson, M., Ni, M., Qin, M. & Quail, P. H. poc1: an Arabidopsis mutant perturbed in phytochrome signalling due to a T-DNA insertion in the promoter of PIF3, a gene encoding a phytochrome-interacting, bHLH protein. Proc. Natl Acad. Sci. USA 96, 5832–5837 (1999).
Huq, E., Kang, Y., Qin, M. & Quail, P. H. SRL1: a new locus specific to the phyB signalling pathway in Arabidopsis. Plant J. 23, 1–11 (2000).
Ullah, H. et al. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292, 2066–2069 (2001).
Sakamoto, K. & Nagatani, A. Nuclear localization activity of phytochrome B. Plant J. 10, 859–868 (1996).
Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S. A. & Nagatani, A. Light-dependent translocation of a phytochrome B–GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 145, 437–445 (1999).
Kircher, S. et al. Light quality-dependent nuclear import of the plant photoreceptors phytochrome A and B. Plant Cell 11, 1445–1456 (1999). Together with references 48 and 49 , this paper provides the initial evidence that phytochromes are induced to translocate from the cytoplasm to the nucleus on photoconversion to the biologically active Pfr conformer.
Hisada, A. et al. Light-induced nuclear translocation of endogenous pea phytochrome A visualized by immunocytochemical procedures. Plant Cell 12, 1063–1078 (2000).
Kim, L. et al. Light-induced nuclear import of phytochrome-A:GFP fusion proteins is differentially regulated in transgenic tobacco and Arabidopsis. Plant J. 22, 125–133 (2000).
Nagy, F. & Schäfer, E. Nuclear and cytosolic events of light-induced, phytochrome-regulated signalling in higher plants. EMBO J. 19, 157–163 (2000).
Nagy, F. & Schäfer, E. Control of nuclear import and phytochromes. Curr. Opin. Plant Biol. 3, 450–454 (2000).
Nagy, F., Kircher, S. & Schäfer, E. Nucleo-cytoplasmic partitioning of the plant photoreceptor phytochromes. Semin. Cell Dev. Biol. 11, 505–510 (2000).
Gil, P. et al. Photocontrol of subcellular partitioning of phytochrome-B:GFP fusion protein in tobacco seedlings. Plant J. 22, 135–145 (2000).
Mas, P., Devlin, P. F., Panda, S. & Kay, S. A. Functional interaction of phytochrome B and cryptochrome 2. Nature 408, 207–211 (2000).
Tepperman, J. M., Zhu, T., Chang, H.-S., Wang, X. & Quail, P. H. Multiple transcription-factor genes are early targets of phytochrome A signalling. Proc. Natl Acad. Sci. USA 98, 9437–9442 (2001). A microarray-based, time-course analysis of expression profiles in wild-type versus phyA -null mutants of Arabidopsis provides evidence that a master set of genes that encode a spectrum of different classes of transcription factors are potential primary targets of phyA signalling.
Fankhauser, C. et al. PKS1, a substrate phosphorylated by phytochrome that modulates light signalling in Arabidopsis. Science 284, 1539–1541 (1999). This study provides the first evidence that the Ser/Thr protein kinase activity of recombinant phytochrome preparations is capable of in vitro light-regulated, differential transphosphorylation of a protein substrate, PKS1, which was originally isolated as a phyA interactor in a yeast two-hybrid screen.
Choi, G. et al. Phytochrome signalling is mediated through nucleoside diphosphate kinase 2. Nature 401, 610–613 (1999).
Ni, M., Tepperman, J. M. & Quail, P. H. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400, 781–784 (1999).
Zhu, Y., Tepperman, J. M., Fairchild, C. D. & Quail, P. Phytochrome B binds with greater apparent affinity than phytochrome A to the basic helix-loop-helix factor PIF3 in a reaction requiring the PAS domain of PIF3. Proc. Natl Acad. Sci. USA 97, 13419–13424 (2000).
Martínez-García, J. F., Huq, E. & Quail, P. H. Direct targeting of light signals to a promoter element-bound transcription factor. Science 288, 859–863 (2000). This study provides evidence that phyB in its biologically active Pfr conformer can bind specifically, and photoreversibly, to the bHLH transcription factor, PIF3, which is already bound to its cognate G-box DNA-binding site found in the promoters of various phy-regulated genes.
Wang, Z.-Y. et al. A myb-related transcription factor is involved in the phytochrome regulation of an Arabidopsis Lhcb gene. Plant Cell 9, 491–507 (1997).
Schaffer, R. et al. The late elongated hypocotyl mutation of Arabidopsis disrupts circadian rhythms and the photoperiodic control of flowering. Cell 93, 1219–1229 (1998).
Wang, Z.-Y. & Tobin, E. M. Constitutive expression of the Circadian clock associated 1 (CCA1) gene disrupts circadian rhythms and suppresses its own expression. Cell 93, 1207–1217 (1998).
Green, R. M. & Tobin, E. M. Loss of the circadian clock-associated protein 1 in Arabidopsis results in altered clock-regulated gene expression. Proc. Natl Acad. Sci. USA 96, 4176–4179 (1999).
Harmer, S. L. et al. Orchestrated transcription of key pathways in Arabidopsis by the circadian clock. Science 290, 2110–2113 (2000).
Alabadi, D. et al. Reciprocal regulation between TOC1 and LHY/CCA1 within the Arabidopsis circadian clock. Science 293, 880–883 (2001).
Ahmad, M., Jarillo, J. A., Smirnova, O. & Cashmore, A. R. The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell 1, 939–948 (1998).
Colon-Carmona, A., Chen, D. L., Yeh, K. C., & Abel, S. Aux/IAA proteins are phosphorylated by phytochrome in vitro. Plant Physiol. 124, 1728–1738 (2000).
Jarillo, J. A. et al. An Arabidopsis circadian clock component interacts with both CRY1 and phyB. Nature 410, 487–490 (2001).
Sweere, U. et al. Interaction of the response regulator ARR4 with the photoreceptor phytochrome B in modulating red light signalling. Science 294, 1108–1111 (2001).
Fankhauser, C. Phytochromes as light-modulated protein kinases. Semin. Cell Dev. Biol. 11, 467–473 (2000).
Hanks, S. K. & Quinn, A. M. In Protein Phosphorylation A. Protein Kinases: Assays, Purification, Antibodies, Functional Analysis, Cloning, and Expression (eds Hunter, T. & Sefton, B. M.) 38–62 (Academic Press, San Diego, California, 1991).
Lagarias, J. C., Wong, Y.-S., Berkelman, T. R., Kidd, D. G. & McMichael, R. W. Jr. Structure–function studies on Avena phytochrome. In Phytochrome and Photoregulation in Plants (ed Furuya, M.) 51–62 (Academic Press, Tokyo, 1987).
McMichael, R. W. Jr & Lagarias, J. C. Phosphopeptide mapping of Avena phytochrome phosphorylated by protein kinases in vitro. Biochemistry 29, 3872–3878 (1990).
Wong, Y.-S., McMichael, R. W. Jr & Lagarias, J. C. Properties of a polycation-stimulated protein kinase associated with purified Avena phytochrome. Plant Physiol. 91, 709–718 (1989).
Yeh, K.-C. & Lagarias, J. C. Eukaryotic phytochromes: light-regulated serine/theonine protein kinases with histidine kinase ancestry. Proc. Natl Acad. Sci. USA 95, 13976–13981 (1998).
Hoch, J. A. & Silhavy, T. J. Two-Component Signal Transduction (Am. Soc. Microbiol. Press, Washington DC., 1995).
Schneider-Poetsch, H. A. W. Signal transduction by phytochrome: phytochromes have a module related to the transmitter modules of bacterial sensor proteins. Photochem. Photobiol. 56, 839–846 (1992).
Schneider-Poetsch, H. A. W. & Braun, B. Proposal on the nature of phytochrome action based on the carboxy-terminal sequences of phytochrome. J. Plant Physiol. 137, 576–580 (1991).
Schneider-Poetsch, H. A. W., Braun, B., Marx, S. & Schaumburg, A. Phytochromes and bacterial sensor proteins are related by structural and functional homologies. FEBS J. 281, 245–249 (1991).
Kaneko, T. et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3, 109–136 (1996).
Appleby, J. L. & Bourret, R. B. Activation of CheY mutant D57N by phosphorylation at an alternative site, Ser-56. Mol. Microbiol. 34, 915–925 (1999).
Osterlund, M. T., Ang, L.-H. & Deng, X.-W. The role of COP1 in repression of Arabidopsis photomorphogenic development. Trends Cell Biol. 9, 113–118 (1999).
Osterlund, M. T., Hardtke, C. S., Wei, N. & Deng, X. W. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405, 462–466 (2000). This study provides evidence that the abundance of the bZIP transcription factor, HY5, with an established role in photomorphogenic development, is regulated by light at the level of protein degradation through a proteasome pathway.
Wei, N. & Deng, X. W. Making sense of the COP9 signalosome — a regulatory protein complex conserved from Arabidopsis to human. Trends Genet. 15, 98–103 (1999).
Schwechheimer, C. & Deng, X. W. COP9 signalosome revisited: a novel mediator of protein degradation. Trends Cell Biol. 11, 420–426 (2001).
Schwechheimer, C. et al. Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 292, 1379–1382 (2001).
Wang, H. Y., Ma, L. G., Li, J. M., Zhao, H. Y. & Deng, X. W. Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294, 154–158 (2001).
Yang, H.-Q., Tang, R.-H. & Cashmore, A. R. The signalling mechanism of Arabidopsis CRY1 involves direct interaction with COP1. Plant Cell 13, 2573–2587 (2001).
Hoecker, U. & Quail, P. H. The phytochrome A-specific signaling intermediate SPA1 interacts directly with COP1, a constitutive repressor of light signaling in Arabidopsis. J. Biol. Chem. 276, 38173–38178 (2001).
Okamoto, H., Qu, L. & Deng, X.-W. Does EID1 aid the fine-tuning of phytochrome A signal transduction in Arabidopsis? Plant Cell 13, 1983–1986 (2001).
von Arnim, A., Osterlund, M. T., Kwok, S. F. & Deng, X.-W. Genetic and developmental control of nuclear accumulation of COP1, a repressor or photomorphogenesis in Arabidopsis. Plant Physiol. 114, 779–788 (1997).
Ma, L. et al. Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13, 2589–2607 (2001).
Bolle, C., Koncz, C. & Chua, N. H. PAT1, a new member of the GRAS family, is involved in phytochrome A signal transduction. Genes Dev. 14, 1269–1278 (2000).
Hsieh, H.-L. et al. Fin219, an auxin-regulated gene, defines a link between phytochrome A and the downstream regulator COP1 in light control of Arabidopsis development. Genes Dev. 14, 1958–1970 (2000).
Desnos, T., Puente, P., Whitelam, G. C. & Harberd, N. P. FHY1: a phytochrome A-specific signal transducer. Genes Dev. 15, 2980–2990 (2001).
Oyama, T., Shimura, Y. & Okada, K. The Arabidopsis HY5 gene encodes a bZIP protein that regulates stimulus-induced development of root and hypocotyl. Genes Dev. 11, 2983–2995 (1997).
Ahmad, M. & Cashmore, A. R. The pef mutants of Arabidopsis thaliana define lesions early in the phytochrome signaling pathway. Plant J. 10, 1103–1110 (1996).
Genoud, T. et al. An Arabidopsis mutant hypersensitive to red and far-red light signals. Plant Cell 10, 889–904 (1998).
Wagner, D., Hoecker, E. & Quail, P. H. RED1 is necessary for phytochrome B-mediated red light specific signal transduction in Arabidopsis. Plant Cell 9, 731–743 (1997).
Whitelam, G. C. et al. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5, 757–768 (1993).
Acknowledgements
I thank the members of my laboratory for stimulating discussions; E. Huq and M. Hudson for helpful comments on the manuscript; E. Schäfer and S. Kircher for the images in Figure 3; J. Tepperman for preparing the figures; and R. Wells for manuscript preparation and editing. Research is supported by grants from: National Institutes of Health, United States Department of Energy, Torrey Mesa Research Institute, Syngenta Research Technology and United States Department of Agriculture.
Author information
Authors and Affiliations
Glossary
- PHOTOMORPHOGENESIS
-
Changes in growth and development in response to light. These occur throughout the plant life cycle, from seed germination, and seedling de-etiolation, through vegetative architectural adaptations and flower induction, and are observable at all levels of organization, from visible phenotype to gene expression.
- CHROMOPROTEIN
-
A molecule that consists of two moieties: a polypeptide and a chromophore, the latter of which absorbs light of specific wavelengths and causes the molecule to be coloured.
- Pr
-
The biologically inactive form of the phytochrome molecule that absorbs red light (P for phytochrome; r for red-light-absorbing).
- Pfr
-
The biologically active form of the phytochrome molecule that absorbs far-red light (P for phytochrome; fr for far-red-absorbing).
- HYPOCOTYL
-
The equivalent of the stem in a young seedling. The vegetative organ between the root and the cotyledons, which is responsible for initial upward elongation growth.
- COTYLEDONS
-
Referred to as 'seed leaves', these organs initially remain small and yellow in colour, and provide storage reserves for seedling growth in darkness (heterotrophic growth), but rapidly expand and turn green in response to light as chloroplasts develop and photosynthesis begins (autotrophic growth).
- F-BOX PROTEIN
-
A class of protein that contains a conserved sequence motif referred to as an F-box domain. The particular F-box variant in a given SCF-type E3-ubiquitin-ligase complex confers target-protein substrate specificity on the SCF complex.
- SCF-TYPE E3 UBIQUITIN LIGASE
-
Enzyme complex that catalyses the ubiquitylation of target proteins. The complex contains three main components, termed Skp1, a member of the cullin family (Cul1) and F-box proteins.
Rights and permissions
About this article
Cite this article
Quail, P. Phytochrome photosensory signalling networks. Nat Rev Mol Cell Biol 3, 85–93 (2002). https://doi.org/10.1038/nrm728
Issue Date:
DOI: https://doi.org/10.1038/nrm728
This article is cited by
-
Optical properties of Sr2LuNbO6:Mn4+ deep-red phosphor: crystal-field splitting and multimode vibrational coupling
Science China Materials (2024)
-
A 5.5-kb LTR-retrotransposon insertion inside phytochrome B gene (CsPHYB) results in long hypocotyl and early flowering in cucumber (Cucumis sativus L.)
Theoretical and Applied Genetics (2023)
-
Different combinations of red and blue LED light affect the growth, physiology metabolism and photosynthesis of in vitro-cultured Dendrobium nobile ‘Zixia’
Horticulture, Environment, and Biotechnology (2023)
-
Arabidopsis cryptochrome 2 forms photobodies with TCP22 under blue light and regulates the circadian clock
Nature Communications (2022)
-
Ultrafast proton-coupled isomerization in the phototransformation of phytochrome
Nature Chemistry (2022)