Key Points
-
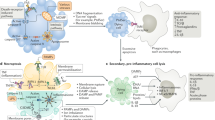
Mitochondria not only generate the vast majority of intracellular ATP but also receive and decode organelle-extrinsic signals of stress, such as those generated during viral infection, to activate adaptive responses.
-
When homeostasis cannot be re-established, mitochondrial membranes become permeabilized, which leads to the activation of caspase-dependent and caspase-independent cell death effector mechanisms. Mitochondria regulate intrinsic apoptosis, have a prominent role in multiple instances of extrinsic apoptosis and are involved in the execution of regulated necrosis.
-
Mitochondria are capable of emitting a wide array of danger signals that alert the cell of perturbations in homeostasis. These signals include mitochondrial DNA (mtDNA), reactive oxygen species (ROS) and specific nucleus-encoded proteins, all of which activate adaptive programmes aimed at recovering mitochondrial functions.
-
In conditions of extensive tissue damage, these and other mitochondrial danger signals, such as N-formyl-peptides (NFPs), are released into the extracellular space and eventually reach the bloodstream, where they can stimulate widespread innate immune responses that can contribute to the pathophysiology of life-threatening conditions such as systemic inflammatory response syndrome (SIRS).
-
Thus, mitochondria can be considered as master regulators of cellular and systemic danger signalling.
Abstract
Throughout more than 1.5 billion years of obligate endosymbiotic co-evolution, mitochondria have developed not only the capacity to control distinct molecular cascades leading to cell death but also the ability to sense (and react to) multiple situations of cellular stress, including viral infection. In addition, mitochondria can emit danger signals that alert the cell or the whole organism of perturbations in homeostasis, hence promoting the induction of cell-intrinsic or systemic adaptive responses, respectively. As such, mitochondria can be considered as master regulators of danger signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kroemer, G. Mitochondrial implication in apoptosis. Towards an endosymbiont hypothesis of apoptosis evolution. Cell Death Differ. 4, 443–456 (1997).
Hengartner, M. O., Ellis, R. E. & Horvitz, H. R. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature 356, 494–499 (1992).
Kroemer, G., Galluzzi, L. & Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87, 99–163 (2007). A comprehensive overview of the crucial role of MOMP in cell death.
Galluzzi, L. et al. Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 19, 107–120 (2012).
Tait, S. W. & Green, D. R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nature Rev. Mol. Cell Biol. 11, 621–632 (2010).
Kroemer, G., Marino, G. & Levine, B. Autophagy and the integrated stress response. Mol. Cell 40, 280–293 (2010).
Gomes, L. C., Di Benedetto, G. & Scorrano, L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nature Cell Biol. 13, 589–598 (2011). Demonstrates that during autophagy a population of mitochondria is spared from degradation and promotes cell survival by ensuring ATP supplies.
Tondera, D. et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 28, 1589–1600 (2009).
Arnoult, D., Soares, F., Tattoli, I. & Girardin, S. E. Mitochondria in innate immunity. EMBO Rep. 12, 901–910 (2011).
Krysko, D. V. et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. 32, 157–164 (2011).
Zhang, Q. et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464, 104–107 (2010). Shows that circulating mitochondrial components, including mtDNA and NFPs, trigger a massive activation of neutrophils and hence promote multiorgan injury and SIRS.
Oka, T. et al. Mitochondrial DNA that escapes from autophagy causes inflammation and heart failure. Nature 485, 251–255 (2012).
Zamzami, N. et al. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 181, 1661–1672 (1995). The first demostration that MOMP constitutes an early and irreversible step in the cascade of events leading to cell death.
Galluzzi, L., Kepp, O., Trojel-Hansen, C. & Kroemer, G. Non-apoptotic functions of apoptosis-regulatory proteins. EMBO Rep. 13, 322–330 (2012).
Jost, P. J. et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature 460, 1035–1039 (2009).
Vandenabeele, P., Galluzzi, L., Vanden Berghe, T. & Kroemer, G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nature Rev. Mol. Cell Biol. 11, 700–714 (2010).
Vitale, I., Galluzzi, L., Castedo, M. & Kroemer, G. Mitotic catastrophe: a mechanism for avoiding genomic instability. Nature Rev. Mol. Cell Biol. 12, 385–392 (2011).
Wang, Z., Jiang, H., Chen, S., Du, F. & Wang, X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell 148, 228–243 (2012).
Galluzzi, L., Kepp, O., Trojel-Hansen, C. & Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 111, 1198–1207 (2012).
Hornung, V. et al. 5′-triphosphate RNA is the ligand for RIG-I. Science 314, 994–997 (2006).
Pichlmair, A. et al. RIG-I-mediated antiviral responses to single-stranded RNA bearing 5′-phosphates. Science 314, 997–1001 (2006).
Zeng, W. et al. Reconstitution of the RIG-I pathway reveals a signaling role of unanchored polyubiquitin chains in innate immunity. Cell 141, 315–330 (2010).
Seth, R. B., Sun, L., Ea, C. K. & Chen, Z. J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-κB and IRF3. Cell 122, 669–682 (2005).
Liu, H. M. et al. The mitochondrial targeting chaperone 14-3-3ε regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe 11, 528–537 (2012).
Hou, F. et al. MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011).
Xu, L. G. et al. VISA is an adapter protein required for virus-triggered IFN-β signaling. Mol. Cell 19, 727–740 (2005).
Paz, S. et al. A functional C-terminal TRAF3-binding site in MAVS participates in positive and negative regulation of the IFN antiviral response. Cell Res. 21, 895–910 (2011).
Allen, I. C. et al. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I–MAVS and TRAF6–NF-κB signaling pathways. Immunity 34, 854–865 (2011).
Lei, Y. et al. The mitochondrial proteins NLRX1 and TUFM form a complex that regulates type I interferon and autophagy. Immunity 36, 933–946 (2012).
Levine, B., Mizushima, N. & Virgin, H. W. Autophagy in immunity and inflammation. Nature 469, 323–335 (2011).
Xu, L., Xiao, N., Liu, F., Ren, H. & Gu, J. Inhibition of RIG-I and MDA5-dependent antiviral response by gC1qR at mitochondria. Proc. Natl Acad. Sci. USA 106, 1530–1535 (2009).
Lin, R., Paz, S. & Hiscott, J. Tom70 imports antiviral immunity to the mitochondria. Cell Res. 20, 971–973 (2010).
Yang, K. et al. Hsp90 regulates activation of interferon regulatory factor 3 and TBK-1 stabilization in Sendai virus-infected cells. Mol. Biol. Cell 17, 1461–1471 (2006).
Onoguchi, K. et al. Virus-infection or 5′ ppp-RNA activates antiviral signal through redistribution of IPS-1 mediated by MFN1. PLoS Pathog. 6, e1001012 (2010).
Yasukawa, K. et al. Mitofusin 2 inhibits mitochondrial antiviral signaling. Sci. Signal. 2, ra47 (2009).
Koshiba, T., Yasukawa, K., Yanagi, Y. & Kawabata, S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci. Signal. 4, ra7 (2011).
Belgnaoui, S. M., Paz, S. & Hiscott, J. Orchestrating the interferon antiviral response through the mitochondrial antiviral signaling (MAVS) adapter. Curr. Opin. Immunol. 23, 564–572 (2011).
Raturi, A. & Simmen, T. Where the endoplasmic reticulum and the mitochondrion tie the knot: the mitochondria-associated membrane (MAM). Biochim. Biophys. Acta 2 May 2012 (doi:10.1016/j.bbamcr.2012.04.013).
Horner, S. M., Liu, H. M., Park, H. S., Briley, J. & Gale, M. Jr. Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc. Natl Acad. Sci. USA 108, 14590–14595 (2011).
Ishikawa, H. & Barber, G. N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 (2008).
Ishikawa, H., Ma, Z. & Barber, G. N. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461, 788–792 (2009). References 40 and 41 characterize in detail the central function of STING in the signalling cascades that are elicited by viral and bacterial nucleic acids.
Tanaka, Y. & Chen, Z. J. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci. Signal. 5, ra20 (2012).
Lei, Y. et al. MAVS-mediated apoptosis and its inhibition by viral proteins. PLoS ONE 4, e5466 (2009).
Xu, Y., Zhong, H. & Shi, W. MAVS protects cells from apoptosis by negatively regulating VDAC1. Mol. Cell. Biochem. 15 Nov 2010 (doi:10.1007/s11010-010-0658-4).
Ling, Z., Tran, K. C. & Teng, M. N. Human respiratory syncytial virus nonstructural protein NS2 antagonizes the activation of β interferon transcription by interacting with RIG-I. J. Virol. 83, 3734–3742 (2009).
Varga, Z. T., Grant, A., Manicassamy, B. & Palese, P. Influenza virus protein PB1-F2 inhibits the induction of type I interferon by binding to MAVS and decreasing mitochondrial membrane potential. J. Virol. 86, 8359–8366 (2012).
Nunnari, J. & Suomalainen, A. Mitochondria: in sickness and in health. Cell 148, 1145–1159 (2012).
Green, D. R., Galluzzi, L. & Kroemer, G. Mitochondria and the autophagy–inflammation–cell death axis in organismal aging. Science 333, 1109–1112 (2011).
Tait, S. W. & Green, D. R. Mitochondria and cell signalling. J. Cell Sci. 125, 807–815 (2012).
Hamanaka, R. B. & Chandel, N. S. Mitochondrial reactive oxygen species regulate hypoxic signaling. Curr. Opin. Cell Biol. 21, 894–899 (2009).
Al-Mehdi, A. B. et al. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 5, ra47 (2012).
Formentini, L., Sánchez-Arago, M., Sánchez-Cenizo, L. & Cuezva, J. M. The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol. Cell 45, 731–742 (2012).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nature Immunol. 12, 222–230 (2011). Demonstrates that mtDNA promotes the activation of the NLRP3 inflammasome, a process that is maintained under control by the autophagic machinery.
Shimada, K. et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36, 401–414 (2012).
Kroemer, G. et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 16, 3–11 (2009).
Cookson, M. R. & Bandmann, O. Parkinson's disease: insights from pathways. Hum. Mol. Genet. 19, R21–R27 (2010).
Egan, D. F. et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331, 456–461 (2010).
Zhao, Q. et al. A mitochondrial specific stress response in mammalian cells. EMBO J. 21, 4411–4419 (2002).
Haynes, C. M. & Ron, D. The mitochondrial UPR — protecting organelle protein homeostasis. J. Cell Sci. 123, 3849–3855 (2010).
Baker, B. M., Nargund, A. M., Sun, T. & Haynes, C. M. Protective coupling of mitochondrial function and protein synthesis via the eIF2α kinase GCN-2. PLoS Genet. 8, e1002760 (2012).
Rath, E. et al. Induction of dsRNA-activated protein kinase links mitochondrial unfolded protein response to the pathogenesis of intestinal inflammation. Gut 61, 1269–1278 (2012).
Nargund, A. M., Pellegrino, M. W., Fiorese, C. J., Baker, B. M. & Haynes, C. M. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337, 587–590 (2012).
Marques, P. E. et al. Chemokines and mitochondrial products activate neutrophils to amplify organ injury during mouse acute liver failure. Hepatology 56, 1971–1982 (2012).
Hauser, C. J. et al. Mitochondrial damage associated molecular patterns from femoral reamings activate neutrophils through formyl peptide receptors and P44/42 MAP kinase. J. Orthop. Trauma 24, 534–538 (2010).
Hemmi, H. et al. A Toll-like receptor recognizes bacterial DNA. Nature 408, 740–745 (2000).
Yousefi, S. et al. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nature Med. 14, 949–953 (2008).
Cardini, S. et al. Genetic ablation of the Fpr1 gene confers protection from smoking-induced lung emphysema in mice. Am. J. Respir. Cell. Mol. Biol. 47, 332–339 (2012).
McDonald, B. et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330, 362–366 (2010).
Julian, M. W. et al. Mitochondrial transcription factor A serves as a danger signal by augmenting plasmacytoid dendritic cell responses to DNA. J. Immunol. 189, 433–443 (2012).
Zitvogel, L., Kepp, O. & Kroemer, G. Decoding cell death signals in inflammation and immunity. Cell 140, 798–804 (2010).
Sims, G. P., Rowe, D. C., Rietdijk, S. T., Herbst, R. & Coyle, A. J. HMGB1 and RAGE in inflammation and cancer. Annu. Rev. Immunol. 28, 367–388 (2010).
Michaud, M. et al. Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334, 1573–1577 (2011).
Elliott, M. R. et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461, 282–286 (2009).
Schlame, M. Cardiolipin synthesis for the assembly of bacterial and mitochondrial membranes. J. Lipid Res. 49, 1607–1620 (2008).
Dieude, M. et al. Cardiolipin binds to CD1d and stimulates CD1d-restricted γδ T cells in the normal murine repertoire. J. Immunol. 186, 4771–4781 (2011).
Emeagi, P. U. et al. Proinflammatory characteristics of SMAC/DIABLO-induced cell death in antitumor therapy. Cancer Res. 72, 1342–1352 (2012).
Grundtman, C., Kreutmayer, S. B., Almanzar, G., Wick, M. C. & Wick, G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 31, 960–968 (2011).
Alard, J. E. et al. Autoantibodies to endothelial cell surface ATP synthase, the endogenous receptor for Hsp60, might play a pathogenic role in vasculatides. PLoS ONE 6, e14654 (2011).
Calderwood, S. K., Mambula, S. S. & Gray, P. J. Jr. Extracellular heat shock proteins in cell signaling and immunity. Ann. N. Y. Acad. Sci. 1113, 28–39 (2007).
Mookerjee-Basu, J. et al. F1-adenosine triphosphatase displays properties characteristic of an antigen presentation molecule for Vγ9Vδ2 T cells. J. Immunol. 184, 6920–6928 (2010).
Rakoff-Nahoum, S. & Medzhitov, R. Toll-like receptors and cancer. Nature Rev. Cancer 9, 57–63 (2009).
Yang, Z. & Klionsky, D. J. Mammalian autophagy: core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 22, 124–131 (2010).
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Acknowledgements
The authors are supported by the Ligue contre le Cancer (équipe labellisée), AXA Chair for Longevity Research, Cancéropôle Ile-de-France, Institut National du Cancer (INCa), Fondation Bettencourt–Schueller, Fondation de France, Fondation pour la Recherche Médicale, Agence National de la Recherche and the European Commission (Apo-Sys, ArtForce, ChemoRes. Death-Train) and the LabEx Immuno-Oncology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Pattern recognition receptors
-
(PRRs). Host receptors, including Toll-like receptors and NOD-like receptors, that can sense pathogen-associated molecular patterns and initiate signalling cascades that lead to an innate immune response.
- Type I interferon
-
A group of pro-inflammatory cytokines structurally and functionally related to interferon-α that are produced in high levels by cells of the innate immune system in response to viral infection and promote the establishment of a widespread antiviral status.
- Mitochondria-associated membranes
-
(MAMs). Sites of anatomical and functional interconnection between mitochondria and the endoplasmic reticulum.
- CpG
-
Cytosine-guanosine DNA sequence. Unmethylated CpG sequences are prevalent in bacterial DNA but rare in eukaryotic genomes.
- Systemic inflammatory response syndrome
-
(SIRS). A frequently lethal clinical condition that resemble sepsis in its manifestations but does not necessarily involve a microbial component.
- Plasmacytoid dendritic cells
-
(pDCs). A subset of DCs that differ from their conventional (also known as myeloid) counterparts in morphology and in their capacity to produce copious amounts of type I interferon in response to viruses and Toll-like receptor ligands.
- CD1d
-
A glycoprotein expressed on the surface of several antigen-presenting cells that is involved in the presentation of lipid antigens to specific subsets of T cells.
- Antigen-presenting cells
-
(APCs). Crucial cellular components of the adaptive immune response owing to their capacity to process antigens and present antigenic peptides to T cells in the context of appropriate stimulatory signals.
- Immunogenic cell death
-
A functionally unconventional type of apoptosis that results in the activation of an adaptive immune response against antigens from dead cells.
- γδ T cells
-
Type of T cell bearing an unconventional T cell receptor composed of one γ-chain and one δ-chain, rather than one α-chain and one β-chain. Their antigenic repertoire is not yet well characterized, but γδ T cells seem to play a prominent part in the recognition of lipid antigens.
- Sterile inflammation
-
Inflammatory condition that develops in the complete absence of a microbial component.
Rights and permissions
About this article
Cite this article
Galluzzi, L., Kepp, O. & Kroemer, G. Mitochondria: master regulators of danger signalling. Nat Rev Mol Cell Biol 13, 780–788 (2012). https://doi.org/10.1038/nrm3479
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3479
This article is cited by
-
Uncovering impaired mitochondrial and lysosomal function in adipose-derived stem cells from obese individuals with altered biological activity
Stem Cell Research & Therapy (2024)
-
A break in mitochondrial endosymbiosis as a basis for inflammatory diseases
Nature (2024)
-
Association of mitochondrial homeostasis and dynamic balance with malignant biological behaviors of gastrointestinal cancer
Journal of Translational Medicine (2023)
-
Single-cell dissection of aggression in honeybee colonies
Nature Ecology & Evolution (2023)
-
Regulatory effects of trimetazidine in cardiac ischemia/reperfusion injury
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)