Key Points
-
Homologous recombination (HR) has an important role in DNA repair, DNA replication, meiotic chromosome segregation and telomere maintenance. HR is tightly regulated by DNA helicases. A defect in, or deregulation of, HR can lead to cell-cycle arrest, genome destabilization and cancer formation.
-
DNA double-stranded breaks (DSBs) that are caused by exposure to ionizing radiation and other DNA-damaging agents are strong inducers of HR. Programmed HR processes such as meiotic recombination and yeast mating-type switching are initiated through DSB formation.
-
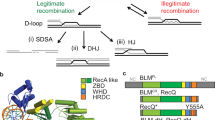
Several HR pathways that are initiated by DSBs have been elucidated. In the DNA double-strand-break repair (DSBR) pathway, a DNA intermediate that contains two Holliday junctions is made. Resolution of this DNA intermediate can produce crossover recombinants that harbour a reciprocal exchange of the arms of the recombining chromosomes.
-
Crossovers are crucial for chromosome segregation at meiosis I, but their formation is suppressed in mitotic cells because of an inherent risk of chromosome rearrangements if the crossovers involve repetitive sequences in the genome.
-
HR is mediated by a class of enzymes known as recombinases. There are two eukaryotic recombinases, RAD51 and DMC1, and both are related to the bacterial recombinase RecA. RAD51 is needed for mitotic and meiotic HR, whereas DMC1 functions only during meiosis.
-
All the recombinases form a helical filament on ssDNA, better known as the presynaptic filament. Assembly of the presynaptic filament requires ATP and is facilitated by recombination mediators, which include the yeast Rad52 protein and the human BRCA2 tumour suppressor.
-
HR is regulated by DNA helicases at several stages. The yeast Mer3 helicase promotes meiotic crossover formation, whereas the yeast Sgs1 and human BLM helicases mediate the dissolution of the double Holliday junction to yield non-crossover recombinants. Moreover, the yeast Srs2 helicase attenuates HR by dismantling the Rad51 presynaptic filament.
-
Many questions concerning the mechanism and regulation of mitotic and meiotic HR remain. For example, how HR functionally synergizes with the Fanconi anaemia protein complex to mediate the removal of interstrand DNA crosslinks remains to be delineated.
Abstract
Homologous recombination (HR) is an important mechanism for the repair of damaged chromosomes, for preventing the demise of damaged replication forks, and for several other aspects of chromosome maintenance. As such, HR is indispensable for genome integrity, but it must be regulated to avoid deleterious events. Mutations in the tumour-suppressor protein BRCA2, which has a mediator function in HR, lead to cancer formation. DNA helicases, such as Bloom's syndrome protein (BLM), regulate HR at several levels, in attenuating unwanted HR events and in determining the outcome of HR. Defects in BLM are also associated with the cancer phenotype. The past several years have witnessed dramatic advances in our understanding of the mechanism and regulation of HR.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Clark, A. J. & Margulies, A. D. Isolation and characterization of recombination-deficient mutants of Escherichia coli K12. Proc. Natl Acad. Sci. USA 53, 451–459 (1965). Reports the first isolation of HR mutants.
Symington, L. S. Role of RAD52 epistasis group genes in homologous recombination and double-strand break repair. Microbiol. Mol. Biol. Rev. 66, 630–670 (2002). A comprehensive review on HR genes, proteins and pathways.
Michel, B., Grompone, G., Flores, M. J. & Bidnenko, V. Multiple pathways process stalled replication forks. Proc. Natl Acad. Sci. USA 101, 12783–12788 (2004).
Paques, F. & Haber, J. E. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 63, 349–404 (1999).
McEachern, M. J. & Haber, J. E. Break-induced replication and recombinational telomere elongation in yeast. Annu. Rev. Biochem. 75, 111–135 (2006). A comprehensive review on BIR pathways and their relevance to telomere maintenance.
Surralles, J. et al. Molecular cross-talk among chromosome fragility syndromes. Genes Dev. 18, 1359–1370 (2004).
Kennedy, R. D. & D'Andrea, A. D. The Fanconi anemia/BRCA pathway: new faces in the crowd. Genes Dev. 19, 2925–2940 (2005).
Hickson, I. D. RecQ helicases: caretakers of the genome. Nature Rev. Cancer 3, 169–178 (2003).
Moynahan, M. E. The cancer connection: BRCA1 and BRCA2 tumour suppression in mice and humans. Oncogene 21, 8994–9007 (2002).
Jasin, M. Homologous repair of DNA damage and tumorigenesis: the BRCA connection. Oncogene 21, 8981–8993 (2002).
Keeney, S. Mechanism and control of meiotic recombination initiation. Curr. Top. Dev. Biol. 52, 1–53 (2001).
Johnson, R. D. & Jasin, M. Double-strand-break-induced homologous recombination in mammalian cells. Biochem. Soc. Trans. 29, 196–201 (2001).
Peterson, C. L. & Cote, J. Cellular machineries for chromosomal DNA repair. Genes Dev. 18, 602–616 (2004).
van Attikum, H. & Gasser, S. M. The histone code at DNA breaks: a guide to repair? Nature Rev. Mol. Cell Biol. 6, 757–765 (2005).
Orr-Weaver, T. L., Szostak, J. W. & Rothstein, R. J. Yeast transformation: a model system for the study of recombination. Proc. Natl Acad. Sci. USA 78, 6354–6358 (1981).
Orr-Weaver, T. L. & Szostak, J. W. Yeast recombination: the association between double-strand gap repair and crossing-over. Proc. Natl Acad. Sci. USA 80, 4417–4421 (1983).
Szostak, J. W., Orr-Weaver, T. L., Rothstein, R. J. & Stahl, F. W. The double-strand-break repair model for recombination. Cell 33, 25–35 (1983). Describes a HR model that is now known as the canonical DSBR model and that can explain meiotic recombination data.
Collins, I. & Newlon, C. S. Meiosis-specific formation of joint DNA molecules containing sequences from homologous chromosomes. Cell 76, 65–75 (1994). Presents the first physical demonstration of meiotic recombination DNA intermediates between homologous chromosomes.
Schwacha, A. & Kleckner, N. Identification of joint molecules that form frequently between homologues but rarely between sister chromatids during yeast meiosis. Cell 76, 51–63 (1994). Presents evidence for DNA joint molecules between non-sister chromatids as recombination intermediates in meiosis.
Schwacha, A. & Kleckner, N. Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 83, 783–791 (1995). Presents evidence that meiotic DNA joint molecules harbour a double HJ.
Ferguson, D. O. & Holloman, W. K. Recombinational repair of gaps in DNA is asymmetric in Ustilago maydis and can be explained by a migrating D-loop model. Proc. Natl Acad. Sci. USA 93, 5419–5424 (1996). Describes a recombination model, which is now known as the SDSA model, that does not entail crossover formation.
Nassif, N., Penney, J., Pal, S., Engels, W. R. & Gloor, G. B. Efficient copying of nonhomologous sequences from ectopic sites via P-element-induced gap repair. Mol. Cell. Biol. 14, 1613–1625 (1994). Reports the elimination of DSBs by SDSA in D. melanogaster.
Strathern, J. N. et al. Homothallic switching of yeast mating type cassettes is initiated by a double-stranded cut in the MAT locus. Cell 31, 183–192 (1982). Discusses how yeast mating-type switching occurs by SDSA.
Hastings, P. J. Recombination in the eukaryotic nucleus. Bioessays 9, 61–64 (1988).
Allers, T. & Lichten, M. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106, 47–57 (2001).
Davis, A. P. & Symington, L. S. RAD51-dependent break-induced replication in yeast. Mol. Cell. Biol. 24, 2344–2351 (2004).
Malkova, A., Naylor, M. L., Yamaguchi, M., Ira, G. & Haber, J. E. RAD51-dependent break-induced replication differs in kinetics and checkpoint responses from RAD51-mediated gene conversion. Mol. Cell. Biol. 25, 933–944 (2005).
Cox, B. S. & Parry, J. M. The isolation, genetics and survival characteristics of ultraviolet light-sensitive mutants in yeast. Mutat. Res. 6, 37–55 (1968).
Snow, R. Mutants of yeast sensitive to ultraviolet light. J. Bacteriol. 94, 571–575 (1967).
Game, J. C. & Mortimer, R. K. A genetic study of X-ray sensitive mutants in yeast. Mutat. Res. 24, 281–292 (1974).
Resnick, M. A. Genetic control of radiation sensitivity in Saccharomyces cerevisiae. Genetics 62, 519–531 (1969).
Nakai, S. & Matsumoto, S. Two types of radiation-sensitive mutant in yeast. Mutat. Res. 4, 129–136 (1967).
Zakharov, I. A., Suslova, N. G. & Fedorova, I. V. Polyploidy and radioresistance in yeast. I. Effect of mutation xrs1–5 on radioresistance of polyploid series of Saccharomyces cerevisiae. Sov. Genet. 6, 1355–1359 (1970).
Bai, Y. & Symington, L. S. A Rad52 homologue is required for RAD51-independent mitotic recombination in Saccharomyces cerevisiae. Genes Dev. 10, 2025–2037 (1996).
Ajimura, M., Leem, S. H. & Ogawa, H. Identification of new genes required for meiotic recombination in Saccharomyces cerevisiae. Genetics 133, 51–66 (1993).
Klein, H. L. RDH54, a RAD54 homologue in Saccharomyces cerevisiae, is required for mitotic diploid-specific recombination and repair and for meiosis. Genetics 147, 1533–1543 (1997).
Shinohara, M. et al. Characterization of the roles of the Saccharomyces cerevisiae RAD54 gene and a homologue of RAD54, RDH54/TID1, in mitosis and meiosis. Genetics 147, 1545–1556 (1997).
Dresser, M. E. et al. DMC1 functions in a Saccharomyces cerevisiae meiotic pathway that is largely independent of the RAD51 pathway. Genetics 147, 533–544 (1997).
Sung, P. Catalysis of ATP-dependent homologous DNA pairing and strand exchange by yeast RAD51 protein. Science 265, 1241–1243 (1994). First demonstration of recombinase activity in the Rad51 protein.
Bishop, D. K., Park, D., Xu, L. & Kleckner, N. DMC1: a meiosis-specific yeast homologue of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell 69, 439–456 (1992).
Yu, X., Jacobs, S. A., West, S. C., Ogawa, T. & Egelman, E. H. Domain structure and dynamics in the helical filaments formed by RecA and Rad51 on DNA. Proc. Natl Acad. Sci. USA 98, 8419–8424 (2001). Compares and contrasts the RecA and Rad51 presynaptic filaments.
Sung, P. & Robberson, D. L. DNA strand exchange mediated by a RAD51–ssDNA nucleoprotein filament with polarity opposite to that of RecA. Cell 82, 453–461 (1995). Shows that Rad51 mediates the recombination reaction via a protein filament that is assembled on ssDNA.
Ogawa, T., Yu, X., Shinohara, A. & Egelman, E. H. Similarity of the yeast RAD51 filament to the bacterial RecA filament. Science 259, 1896–1899 (1993). Presents the first evidence that Rad51 forms a helical protein filament on DNA.
Cox, M. M. The bacterial RecA protein as a motor protein. Annu. Rev. Microbiol. 57, 551–577 (2003).
Bianco, P. R., Tracy, R. B. & Kowalczykowski, S. C. DNA strand exchange proteins: a biochemical and physical comparison. Front. Biosci. 3, D570–D603 (1998). Compares and contrasts the properties of recombinases from different species.
Sung, P. & Stratton, S. A. Yeast Rad51 recombinase mediates polar DNA strand exchange in the absence of ATP hydrolysis. J. Biol. Chem. 271, 27983–27986 (1996).
Menetski, J. P., Bear, D. G. & Kowalczykowski, S. C. Stable DNA heteroduplex formation catalyzed by the Escherichia coli RecA protein in the absence of ATP hydrolysis. Proc. Natl Acad. Sci. USA 87, 21–25 (1990).
Story, R. M., Weber, I. T. & Steitz, T. A. The structure of the E. coli recA protein monomer and polymer. Nature 355, 318–325 (1992).
Wu, Y., Qian, X., He, Y., Moya, I. A. & Luo, Y. Crystal structure of an ATPase-active form of Rad51 homologue from Methanococcus voltae. Insights into potassium dependence. J. Biol. Chem. 280, 722–728 (2005).
Conway, A. B. et al. Crystal structure of a Rad51 filament. Nature Struct. Mol. Biol. 11, 791–796 (2004).
Passy, S. I. et al. Human DMC1 protein binds DNA as an octameric ring. Proc. Natl Acad. Sci. USA 96, 10684–10688 (1999).
Li, Z., Golub, E. I., Gupta, R. & Radding, C. M. Recombination activities of HsDmc1 protein, the meiotic human homologue of RecA protein. Proc. Natl Acad. Sci. USA 94, 11221–11226 (1997).
Masson, J. Y. et al. The meiosis-specific recombinase hDmc1 forms ring structures and interacts with hRad51. EMBO J. 18, 6552–6560 (1999).
Sehorn, M. G., Sigurdsson, S., Bussen, W., Unger, V. M. & Sung, P. Human meiotic recombinase DMC1 promotes ATP-dependent homologous DNA strand exchange. Nature 429, 433–437 (2004). Presents the first evidence that DMC1 mediates the recombination reaction via a helical protein filament assembled on ssDNA.
Lee, M. H. et al. Calcium ion promotes yeast Dmc1 activity via formation of long and fine helical filaments with single-stranded DNA. J. Biol. Chem. 280, 40980–40984 (2005).
Sauvageau, S. et al. Fission yeast Rad51 and Dmc1, two efficient DNA recombinases forming helical nucleoprotein filaments. Mol. Cell. Biol. 25, 4377–4387 (2005).
Bugreev, D. V., Golub, E. I., Stasiak, A. Z., Stasiak, A. & Mazin, A. V. Activation of human meiosis-specific recombinase DMC1 by Ca2+. J. Biol. Chem. 280, 26886–26895 (2005).
Wang, X. & Haber, J. E. Role of Saccharomyces single-stranded DNA-binding protein RPA in the strand invasion step of double-strand break repair. PLoS Biol. 2, E21 (2004).
Sugawara, N., Wang, X. & Haber, J. E. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol. Cell 12, 209–219 (2003). References 59 and 60 examine the temporal order of recruitment of recombination factors to a DNA DSB by chromatin immunoprecipitation.
Wolner, B., van Komen, S., Sung, P. & Peterson, C. L. Recruitment of the recombinational repair machinery to a DNA double-strand break in yeast. Mol. Cell 12, 221–232 (2003).
Sung, P., Krejci, L., Van Komen, S. & Sehorn, M. G. Rad51 recombinase and recombination mediators. J. Biol. Chem. 278, 42729–42732 (2003). A concise review on the enzymology of HR.
Sung, P. Function of yeast Rad52 protein as a mediator between replication protein A and the Rad51 recombinase. J. Biol. Chem. 272, 28194–28197 (1997). This study, together with references 63 and 64, establishes that Rad52 is a recombination mediator for Rad51 recombinase.
Shinohara, A. & Ogawa, T. Stimulation by Rad52 of yeast Rad51-mediated recombination. Nature 391, 404–407 (1998).
New, J. H., Sugiyama, T., Zaitseva, E. & Kowalczykowski, S. C. Rad52 protein stimulates DNA strand exchange by Rad51 and replication protein A. Nature 391, 407–410 (1998).
Sung, P. Yeast Rad55 and Rad57 proteins form a heterodimer that functions with replication protein A to promote DNA strand exchange by Rad51 recombinase. Genes Dev. 11, 1111–1121 (1997). The first report of a recombination mediator for Rad51 recombinase. The Rad55–Rad57 heterodimer mediates the recombination function of Rad51.
Yang, H., Li, Q., Fan, J., Holloman, W. K. & Pavletich, N. P. The BRCA2 homologue Brh2 nucleates RAD51 filament formation at a dsDNA–ssDNA junction. Nature 433, 653–657 (2005). Presents evidence that the BRCA2-like molecule Brh2 has recombination-mediator activity.
San Filippo, J. et al. Recombination mediator and RAD51 targeting activities of a human BRCA2 polypeptide. J. Biol. Chem. 281, 11649–11657 (2006). Presents evidence that a polypeptide that comprises functional domains from human BRCA2 protein has recombination-mediator activity.
Shinohara, A., Ogawa, H. & Ogawa, T. Rad51 protein involved in repair and recombination in S. cerevisiae is a RecA-like protein. Cell 69, 457–470 (1992).
Milne, G. T. & Weaver, D. T. Dominant negative alleles of RAD52 reveal a DNA repair/recombination complex including Rad51 and Rad52. Genes Dev. 7, 1755–1765 (1993).
Mortensen, U. H., Bendixen, C., Sunjevaric, I. & Rothstein, R. DNA strand annealing is promoted by the yeast Rad52 protein. Proc. Natl Acad. Sci. USA 93, 10729–10734 (1996).
Song, B. & Sung, P. Functional interactions among yeast Rad51 recombinase, Rad52 mediator, and replication protein A in DNA strand exchange. J. Biol. Chem. 275, 15895–15904 (2000).
Sugiyama, T. & Kowalczykowski, S. C. Rad52 protein associates with replication protein A (RPA)-single-stranded DNA to accelerate Rad51-mediated displacement of RPA and presynaptic complex formation. J. Biol. Chem. 277, 31663–31672 (2002).
Krejci, L. et al. Interaction with Rad51 is indispensable for recombination mediator function of Rad52. J. Biol. Chem. 277, 40132–40141 (2002).
Sugiyama, T., New, J. H. & Kowalczykowski, S. C. DNA annealing by RAD52 protein is stimulated by specific interaction with the complex of replication protein A and single-stranded DNA. Proc. Natl Acad. Sci. USA 95, 6049–6054 (1998).
Park, M. S., Ludwig, D. L., Stigger, E. & Lee, S. H. Physical interaction between human RAD52 and RPA is required for homologous recombination in mammalian cells. J. Biol. Chem. 271, 18996–19000 (1996).
Yamaguchi-Iwai, Y. et al. Homologous recombination, but not DNA repair, is reduced in vertebrate cells deficient in RAD52. Mol. Cell. Biol. 18, 6430–6435 (1998).
Rijkers, T. et al. Targeted inactivation of mouse RAD52 reduces homologous recombination but not resistance to ionizing radiation. Mol. Cell. Biol. 18, 6423–6429 (1998).
Fujimori, A. et al. Rad52 partially substitutes for the Rad51 paralog XRCC3 in maintaining chromosomal integrity in vertebrate cells. EMBO J. 20, 5513–5520 (2001).
Johnson, R. D. & Symington, L. S. Functional differences and interactions among the putative RecA homologues Rad51, Rad55, and Rad57. Mol. Cell. Biol. 15, 4843–4850 (1995).
Hays, S. L., Firmenich, A. A. & Berg, P. Complex formation in yeast double-strand break repair: participation of Rad51, Rad52, Rad55, and Rad57 proteins. Proc. Natl Acad. Sci. USA 92, 6925–6929 (1995).
Fortin, G. S. & Symington, L. S. Mutations in yeast Rad51 that partially bypass the requirement for Rad55 and Rad57 in DNA repair by increasing the stability of Rad51–DNA complexes. EMBO J. 21, 3160–3170 (2002).
Masson, J. Y. et al. Identification and purification of two distinct complexes containing the five RAD51 paralogs. Genes Dev. 15, 3296–3307 (2001).
Schild, D., Lio, Y. C., Collins, D. W., Tsomondo, T. & Chen, D. J. Evidence for simultaneous protein interactions between human Rad51 paralogs. J. Biol. Chem. 275, 16443–16449 (2000).
Yonetani, Y. et al. Differential and collaborative actions of Rad51 paralog proteins in cellular response to DNA damage. Nucleic Acids Res. 33, 4544–4552 (2005).
Rodrigue, A. et al. Interplay between human DNA repair proteins at a unique double-strand break in vivo. EMBO J. 25, 222–231 (2006).
Sigurdsson, S. et al. Mediator function of the human Rad51B–Rad51C complex in Rad51/RPA-catalyzed DNA strand exchange. Genes Dev. 15, 3308–3318 (2001).
Liu, Y., Masson, J. Y., Shah, R., O'Regan, P. & West, S. C. RAD51C is required for Holliday junction processing in mammalian cells. Science 303, 243–246 (2004).
Sharan, S. K. et al. Embryonic lethality and radiation hypersensitivity mediated by RAD51 in mice lacking BRCA2. Nature 386, 804–810 (1997).
Chen, P. L. et al. The BRC repeats in BRCA2 are critical for RAD51 binding and resistance to methyl methanesulfonate treatment. Proc. Natl Acad. Sci. USA 95, 5287–5292 (1998).
Xia, F. et al. Deficiency of human BRCA2 leads to impaired homologous recombination but maintains normal nonhomologous end joining. Proc. Natl Acad. Sci. USA 98, 8644–8649 (2001). References 90 and 91 establish that HR requires BRCA2.
Moynahan, M. E., Pierce, A. J. & Jasin, M. BRCA2 is required for homology-directed repair of chromosomal breaks. Mol. Cell 7, 263–272 (2001).
Wong, A. K., Pero, R., Ormonde, P. A., Tavtigian, S. V. & Bartel, P. L. RAD51 interacts with the evolutionarily conserved BRC motifs in the human breast cancer susceptibility gene BRCA2. J. Biol. Chem. 272, 31941–31944 (1997).
Yang, H. et al. BRCA2 function in DNA binding and recombination from a BRCA2–DSS1–ssDNA structure. Science 297, 1837–1848 (2002). Describes crystal structures of several fragments of mouse BRCA2 and presents evidence for a DNA-binding function of this protein.
Kojic, M., Kostrub, C. F., Buchman, A. R. & Holloman, W. K. BRCA2 homologue required for proficiency in DNA repair, recombination, and genome stability in Ustilago maydis. Mol. Cell 10, 683–691 (2002).
Sung, P. Mediating repair. Nature Struct. Mol. Biol. 12, 213–214 (2005).
Esashi, F. et al. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature 434, 598–604 (2005).
Tsubouchi, H. & Roeder, G. S. The budding yeast Mei5 and Sae3 proteins act together with Dmc1 during meiotic recombination. Genetics 168, 1219–1230 (2004).
Hayase, A. et al. A protein complex containing Mei5 and Sae3 promotes the assembly of the meiosis-specific RecA homologue Dmc1. Cell 119, 927–940 (2004).
Bishop, D. K. RecA homologues Dmc1 and Rad51 interact to form multiple nuclear complexes prior to meiotic chromosome synapsis. Cell 79, 1081–1092 (1994).
Siaud, N. et al. Brca2 is involved in meiosis in Arabidopsis thaliana as suggested by its interaction with Dmc1. EMBO J. 23, 1392–1401 (2004).
Dray, E., Siaud, N., Dubois, E. & Doutriaux, M. P. Interaction between Arabidopsis Brca2 and its partners Rad51, Dmc1, and Dss1. Plant Physiol. 140, 1059–1069 (2006).
Ferguson, D. O. & Alt, F. W. DNA double strand break repair and chromosomal translocation: lessons from animal models. Oncogene 20, 5572–5579 (2001).
Popescu, N. C. Genetic alterations in cancer as a result of breakage at fragile sites. Cancer Lett. 192, 1–17 (2003).
Richards, R. I. Fragile and unstable chromosomes in cancer: causes and consequences. Trends Genet. 17, 339–345 (2001).
Richardson, C. & Jasin, M. Frequent chromosomal translocations induced by DNA double-strand breaks. Nature 405, 697–700 (2000).
Zhu, C. et al. Unrepaired DNA breaks in p53-deficient cells lead to oncogenic gene amplification subsequent to translocations. Cell 109, 811–821 (2002).
Klein, H. L. A radical solution to death. Nature Genet. 25, 132–134 (2000).
Wu, L. & Hickson, I. D. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature 426, 870–874 (2003). Presents evidence that BLM, the helicase that is mutated in Bloom's syndrome, cooperates with topoisomerase IIIα to dissolve the double HJ structure to yield exclusively non-crossover recombinants.
Klein, H. L. Mutations in recombinational repair and in checkpoint control genes suppress the lethal combination of srs2Δ with other DNA repair genes in Saccharomyces cerevisiae. Genetics 157, 557–565 (2001).
Ira, G., Malkova, A., Liberi, G., Foiani, M. & Haber, J. E. Srs2 and Sgs1–Top3 suppress crossovers during double-strand break repair in yeast. Cell 115, 401–411 (2003). Shows that Srs2 and Sgs1 independently contribute to the suppression of crossover formation in mitotic DSBR by HR.
Gangloff, S., Soustelle, C. & Fabre, F. Homologous recombination is responsible for cell death in the absence of the Sgs1 and Srs2 helicases. Nature Genet. 25, 192–194 (2000).
German, J. Bloom syndrome: a mendelian prototype of somatic mutational disease. Medicine (Baltimore) 72, 393–406 (1993).
Ellis, N. A. et al. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell 83, 655–666 (1995). Presents evidence that Bloom's syndrome is due to the inactivation of a protein that has extensive homology to the E. coli RecQ helicase.
Karow, J. K., Chakraverty, R. K. & Hickson, I. D. The Bloom's syndrome gene product is a 3′–5′ DNA helicase. J. Biol. Chem. 272, 30611–30614 (1997).
Chaganti, R. S., Schonberg, S. & German, J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc. Natl Acad. Sci. USA 71, 4508–4512 (1974).
Rong, L. & Klein, H. L. Purification and characterization of the SRS2 DNA helicase of the yeast Saccharomyces cerevisiae. J. Biol. Chem. 268, 1252–1259 (1993).
Osman, F., Dixon, J., Barr, A. R. & Whitby, M. C. The F-Box DNA helicase Fbh1 prevents Rhp51-dependent recombination without mediator proteins. Mol. Cell. Biol. 25, 8084–8096 (2005).
Morishita, T. et al. Role of the Schizosaccharomyces pombe F-Box DNA helicase in processing recombination intermediates. Mol. Cell. Biol. 25, 8074–8083 (2005).
Aboussekhra, A. et al. RADH, a gene of Saccharomyces cerevisiae encoding a putative DNA helicase involved in DNA repair. Characteristics of radH mutants and sequence of the gene. Nucleic Acids Res. 17, 7211–7219 (1989).
Lawrence, C. W. & Christensen, R. B. Metabolic suppressors of trimethoprim and ultraviolet light sensitivities of Saccharomyces cerevisiae rad6 mutants. J. Bacteriol. 139, 866–876 (1979).
Schiestl, R. H., Prakash, S. & Prakash, L. The SRS2 suppressor of rad6 mutations of Saccharomyces cerevisiae acts by channeling DNA lesions into the RAD52 DNA repair pathway. Genetics 124, 817–831 (1990).
Aguilera, A. & Klein, H. L. Genetic control of intrachromosomal recombination in Saccharomyces cerevisiae. I. Isolation and genetic characterization of hyper-recombination mutations. Genetics 119, 779–790 (1988).
Rong, L., Palladino, F., Aguilera, A. & Klein, H. L. The hyper-gene conversion hpr5–1 mutation of Saccharomyces cerevisiae is an allele of the SRS2/RADH gene. Genetics 127, 75–85 (1991).
Kaytor, M. D., Nguyen, M. & Livingston, D. M. The complexity of the interaction between RAD52 and SRS2. Genetics 140, 1441–1442 (1995).
Milne, G. T., Ho, T. & Weaver, D. T. Modulation of Saccharomyces cerevisiae DNA double-strand break repair by SRS2 and RAD51. Genetics 139, 1189–1199 (1995).
Schild, D. Suppression of a new allele of the yeast RAD52 gene by overexpression of RAD51, mutations in srs2 and ccr4, or mating-type heterozygosity. Genetics 140, 115–127 (1995).
Aboussekhra, A., Chanet, R., Adjiri, A. & Fabre, F. Semidominant suppressors of Srs2 helicase mutations of Saccharomyces cerevisiae map in the RAD51 gene, whose sequence predicts a protein with similarities to procaryotic RecA proteins. Mol. Cell. Biol. 12, 3224–3234 (1992).
Chanet, R., Heude, M., Adjiri, A., Maloisel, L. & Fabre, F. Semidominant mutations in the yeast Rad51 protein and their relationships with the Srs2 helicase. Mol. Cell. Biol. 16, 4782–4789 (1996).
Krejci, L. et al. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature 423, 305–309 (2003). Together with reference 131, this paper documents the capability of Srs2 to dismantle the Rad51 presynaptic filament.
Krejci, L. et al. Role of ATP hydrolysis in the antirecombinase function of Saccharomyces cerevisiae Srs2 protein. J. Biol. Chem. 279, 23193–23199 (2004).
Veaute, X. et al. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature 423, 309–312 (2003).
Papouli, E. et al. Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol. Cell 19, 123–133 (2005). Shows the recruitment of Srs2 to DNA replication forks via its association with SUMO-modified PCNA.
Pfander, B., Moldovan, G. L., Sacher, M., Hoege, C. & Jentsch, S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature 436, 428–433 (2005). Furnishes evidence that Srs2 binds SUMO-modified PCNA, and that this association is important for the prevention of unwanted recombination during S phase.
Aylon, Y., Liefshitz, B., Bitan-Banin, G. & Kupiec, M. Molecular dissection of mitotic recombination in the yeast Saccharomyces cerevisiae. Mol. Cell. Biol. 23, 1403–1417 (2003).
Vaze, M. B. et al. Recovery from checkpoint- mediated arrest after repair of a double-strand break requires Srs2 helicase. Mol. Cell 10, 373–385 (2002).
Liu, Y. & West, S. C. Happy Hollidays: 40th anniversary of the Holliday junction. Nature Rev. Mol. Cell Biol. 5, 937–944 (2004).
Mankouri, H. W. & Hickson, I. D. Understanding the roles of RecQ helicases in the maintenance of genome integrity and suppression of tumorigenesis. Biochem. Soc. Trans. 32, 957–958 (2004).
Liberi, G. et al. Rad51-dependent DNA structures accumulate at damaged replication forks in sgs1 mutants defective in the yeast ortholog of BLM RecQ helicase. Genes Dev. 19, 339–350 (2005).
Wu, L. et al. BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates. Proc. Natl Acad. Sci. USA 103, 4068–4073 (2006).
Raynard, S., Bussen, W. & Sung, P. A double Holliday junction dissolvasome comprising BLM, topoisomerase IIIα, and BLAP75. J. Biol. Chem. 281, 13861–13864 (2006).
Rockmill, B., Fung, J. C., Branda, S. S. & Roeder, G. S. The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Curr. Biol. 13, 1954–1962 (2003).
McVey, M., Larocque, J. R., Adams, M. D. & Sekelsky, J. J. Formation of deletions during double-strand break repair in Drosophila DmBlm mutants occurs after strand invasion. Proc. Natl Acad. Sci. USA 101, 15694–15699 (2004).
Adams, M. D., McVey, M. & Sekelsky, J. J. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science 299, 265–267 (2003).
Nakagawa, T. & Ogawa, H. The Saccharomyces cerevisiae MER3 gene, encoding a novel helicase-like protein, is required for crossover control in meiosis. EMBO J. 18, 5714–5723 (1999). Shows that the MER3 gene exerts crossover control in meiosis.
Nakagawa, T. & Kolodner, R. D. The MER3 DNA helicase catalyzes the unwinding of Holliday junctions. J. Biol. Chem. 277, 28019–28024 (2002).
Mazina, O. M., Mazin, A. V., Nakagawa, T., Kolodner, R. D. & Kowalczykowski, S. C. Saccharomyces cerevisiae Mer3 helicase stimulates 3′–5′ heteroduplex extension by Rad51; implications for crossover control in meiotic recombination. Cell 117, 47–56 (2004). Provides evidence that Mer3 helicase influences the activity of Rad51 recombinase in a manner that might promote crossover formation.
Paull, T. T., Cortez, D., Bowers, B., Elledge, S. J. & Gellert, M. Direct DNA binding by Brca1. Proc. Natl Acad. Sci. USA 98, 6086–6091 (2001).
Hashizume, R. et al. The RING heterodimer BRCA1–-BARD1 is a ubiquitin ligase inactivated by a breast cancer-derived mutation. J. Biol. Chem. 276, 14537–14540 (2001).
Lee, M., Daniels, M. J. & Venkitaraman, A. R. Phosphorylation of BRCA2 by the Polo-like kinase Plk1 is regulated by DNA damage and mitotic progression. Oncogene 23, 865–872 (2004).
Lin, H. R., Ting, N. S., Qin, J. & Lee, W. H. M phase-specific phosphorylation of BRCA2 by Polo-like kinase 1 correlates with the dissociation of the BRCA2-P–CAF complex. J. Biol. Chem. 278, 35979–35987 (2003).
Marston, N. J. et al. Interaction between the product of the breast cancer susceptibility gene BRCA2 and DSS1, a protein functionally conserved from yeast to mammals. Mol. Cell. Biol. 19, 4633–4642 (1999).
Howlett, N. G. et al. Biallelic inactivation of BRCA2 in Fanconi anemia. Science 297, 606–609 (2002).
Schlacher, K. et al. DNA polymerase V and RecA protein, a minimal mutasome. Mol. Cell 17, 561–572 (2005).
Lieber, M. R., Ma, Y., Pannicke, U. & Schwarz, K. Mechanism and regulation of human non-homologous DNA end-joining. Nature Rev. Mol. Cell Biol. 4, 712–720 (2003).
Wesoly, J. et al. Differential contributions of mammalian Rad54 paralogs to recombination, DNA damage repair, and meiosis. Mol. Cell. Biol. 26, 976–989 (2006).
Cheok, C. F. et al. Roles of the Bloom's syndrome helicase in the maintenance of genome stability. Biochem. Soc. Trans. 33, 1456–1459 (2005).
Sehorn, M. G. & Sung, P. Meiotic recombination: an affair of two recombinases. Cell Cycle 3, 1375–1377 (2004).
Chen, Y. K. et al. Heterodimeric complexes of Hop2 and Mnd1 function with Dmc1 to promote meiotic homologue juxtaposition and strand assimilation. Proc. Natl Acad. Sci. USA 101, 10572–10577 (2004).
Petukhova, G. V. et al. The Hop2 and Mnd1 proteins act in concert with Rad51 and Dmc1 in meiotic recombination. Nature Struct. Mol. Biol. 12, 449–453 (2005).
Acknowledgements
We are grateful to M. Sehorn for providing the electron micrographs in Figure 4 and to J. San Filippo and W. Bussen for their help in preparing the figures. We apologize to colleagues whose work could not be cited because of space limitations. The studies in the laboratories of the authors have been supported by research grants from the US National Institutes of Health, the US Department of Defense and the Susan G. Komen Breast Cancer Foundation.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Glossary
- Meiosis I
-
The successful completion of meiosis requires two cell divisions. Meiosis I refers to the first division in which the pairs of homologous chromosomes are segregated into the two daughter cells.
- Fanconi anaemia
-
(FA). A genetically inherited anaemia that leads to bone marrow failure. Patients with FA are also susceptible to acute myelogenous leukaemia and squamous cell carcinomas in multiple organs. The disease is genetically complex.
- Mating-type switching
-
Haploid S. cerevisiae cells can be of one of two mating types. Only cells of opposite mating types can mate to form a diploid. Cells can switch their mating type through an HR-dependent process.
- Crossover
-
One of the possible outcomes of a physical exchange between duplex DNA molecules. Crossover can occur between sister chromatids or between the non-sister chromatids of a homologous pair of chromosomes. Crossover between non-sister chromatids results in new combinations of parental alleles on the crossover chromosomes.
- DNA helicase
-
An enzyme that uses the energy from ATP hydrolysis to separate the two DNA strands in a double helix.
- Post-replication DNA repair
-
A process that repairs gaps on newly replicated DNA using a DNA polymerase. The gaps usually occur opposite adducts or other lesions on the template strand. Post-replication repair fills in the gaps but does not remove the lesions from the template strand DNA.
- Endonuclease
-
An enzyme that catalyses hydrolytic cleavage of DNA in the middle of a DNA strand or a double helix.
- Chromatin immunoprecipitation
-
A technique for determining whether a protein binds to a particular region of the genome in vivo. It involves treating live cells with formaldehyde to form nonspecific crosslinks between the DNA and any associated proteins. The cells are then lysed, the genomic DNA is sheared into small fragments and the protein of interest is immunoprecipitated. Any protein-associated DNA is then removed and analysed by PCR.
- Holliday junction
-
A cruciform DNA structure that is generated during the synaptic phase of homologous recombination. It is named after Robin Holliday, who proposed its existence in 1964.
- Epistasis group
-
A group of genes that are most frequently defined by double-mutant analyses and function in the same biological pathway.
- Orthologue
-
The structural and functional equivalent of a gene or protein in a different species.
- Paralogue
-
A protein that shares some relatedness in sequence with another protein but not necessarily in function. Paralogues arise through gene duplication.
- Loss of heterozygosity
-
(LOH). Loss of biallelic information at a gene or chromosome region through a number of events, including homologous recombination and deletions. LOH can reveal recessive mutations and is often associated with tumours.
- DNA-damage checkpoint
-
A signal-transduction response that is triggered by DNA damage and that results in the arrest (or delay) of cell-cycle progression.
- Translesion DNA polymerase
-
A specialized low-fidelity DNA polymerase that is capable of synthesizing past lesions in the DNA template.
- Topoisomerase
-
An enzyme that removes torsional stress from double-stranded DNA by changing the DNA supercoiling. It accomplishes this goal by breaking and rejoining one, or both, of the DNA strands, thereby inserting or removing superhelical twists.
- Hemicatenane
-
A DNA structure that forms when one strand of a DNA duplex is wound around a strand from another duplex DNA molecule. Newly replicated sister chromatids are thought to harbour hemicatenanes.
- Ubiquitin ligase
-
An enzyme that facilitates the ligation of ubiquitin to target protein substrates by acting as an intermediary between a ubiquitin-conjugating enzyme and a substrate.
Rights and permissions
About this article
Cite this article
Sung, P., Klein, H. Mechanism of homologous recombination: mediators and helicases take on regulatory functions. Nat Rev Mol Cell Biol 7, 739–750 (2006). https://doi.org/10.1038/nrm2008
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2008
This article is cited by
-
Heterologous production of 3-hydroxypropionic acid in Methylorubrum extorquens by introducing the mcr gene via a multi-round chromosomal integration system based on cre-lox71/lox66 and transposon
Microbial Cell Factories (2024)
-
APOBEC3-mediated mutagenesis in cancer: causes, clinical significance and therapeutic potential
Journal of Hematology & Oncology (2023)
-
Targeting of RRM2 suppresses DNA damage response and activates apoptosis in atypical teratoid rhabdoid tumor
Journal of Experimental & Clinical Cancer Research (2023)
-
Transmembrane nuclease NUMEN/ENDOD1 regulates DNA repair pathway choice at the nuclear periphery
Nature Cell Biology (2023)
-
RMI1 facilitates repair of ionizing radiation–induced DNA damage and maintenance of genomic stability
Cell Death Discovery (2023)