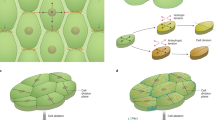
Key Points
-
Cortical microtubules are often, but not always, parallel to the cellulose microfibrils in the cell wall: the alignment of microfibrils helps to regulate the direction in which the cell can expand.
-
Cortical microtubules are nucleated from numerous cortical sites, which are marked by end-binding protein 1 (EB1). The microtubules might be freed by the severing protein katanin and move over the cortex by a modified form of treadmilling, in which there is preferred plus-end assembly.
-
These individual cortical microtubules become organized into characteristic parallel groups, usually transverse to the direction of cell elongation. How these microtubules are ordered in the absence of a centrosome has been an important question.
-
Organization is achieved by a self-ordering process that involves microtubule-associated proteins (MAPs) that are just starting to be described. The filamentous protein MAP65 crossbridges the microtubules into parallel groups; another filament-forming protein, MOR1, also helps regulate microtubule behaviour; a phospholipase D is thought to connect the microtubules to the plasma membrane.
-
Developmental mutants show that the self-organization of the microtubules is important for cells to elongate and plants to develop an axis. The exact relationship between microtubules and cellulose microfibrils during primary growth is still undecided; however, studies on secondary cell-wall formation indicate that intramembranous cellulose-synthesizing enzymes require cortical microtubules, apparently to guide their movement.
Abstract
Plants control the direction of cell expansion as a way of shaping growth. Since their discovery in plants 40 years ago, microtubules have been suspected of forming a template that helps to regulate the direction of growth. The detailed mechanism, however, has been elusive, especially as plants lack a microtubule-organizing centre. Developmental mutants are now beginning to show how microtubules are organized and how this affects plant morphology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Ledbetter, M. C. & Porter, K. R. A 'microtubule' in plant cell fine structure. J. Cell Biol. 19, 239–250 (1963).
Heath, I. & Seagull, R. W. In The Cytoskeleton in Plant Growth and Development (ed. Lloyd, C. W.) 163–182 (Academic, London, 1982).
Lancelle, S. A., Callaham, D. A. & Hepler, P. K. A method for rapid freeze fixation of plant cells. Protoplasma 131, 153–165 (1986)
Lloyd, C. W., Chan, J. & Hussey, P. J. in The Plant Cytoskeleton in Cell Differentiation and Development (ed. Hussey, P. J.) 3–27 (Blackwell, Oxford, 2003).
Schmit, A. C. Acentrosomal microtubule nucleation in higher plants. Int. Rev. Cytol. 220, 257–289 (2002).
Stoppin, V., Vantard, M., Schmit, A. C. & Lambert, A. M. Isolated plant nuclei nucleate microtubule assembly: the nuclear surface in higher plants has centrosome-like activity. Plant Cell 6, 1099–1106 (1994).
Yuan, M., Shaw, P. J., Warn, R. M. & Lloyd, C. W. Dynamic reorientation of cortical microtubules, from transverse to longitudinal, in living plant cells. Proc. Natl Acad. Sci. USA 91, 6050–6053 (1994).
Granger, C. L. & Cyr, R. J. Spatiotemporal relationships between growth and microtubule orientation as revealed in living root cells of Arabidopsis thaliana transformed with green-fluorescent-protein gene construct GFP–MBD. Protoplasma 216, 201–214 (2001).
Shaw, S. L., Kamyar, R. & Ehrhardt, D. W. Sustained microtubule treadmilling in Arabidopsis cortical arrays. Science 300, 1715–1718 (2003). Evidence that cortical microtubules translocate using a modified form of treadmilling.
Dhonukshe, P. & Gadella, T. W. Alteration of microtubule dynamic instability during preprophase band formation revealed by yellow fluorescent protein–CLIP170 microtubule plus-end labeling. Plant Cell 15, 597–611 (2003).
Lloyd, C. Why should stationary plant cells have such dynamic microtubules? Mol. Biol. Cell 5, 1277–1280 (1994).
Job, D., Valiron, O. & Oakley, B. Microtubule nucleation. Curr. Opin. Cell Biol. 15, 111–117 (2003).
Panteris, E., Apostolakos, P., Graf, R. & Galatis, B. γ-tubulin colocalizes with microtubule arrays and tubulin paracrystals in dividing vegetative cells of higher plants. Protoplasma 210, 179–187 (2000).
Drykova, D. et al. Plant γ-tubulin interacts with αβ-tubulin dimers and forms membrane-associated complexes. Plant Cell 15, 465–480 (2003).
Erhardt, M. et al. The plant Spc98p homologue colocalizes with γ-tubulin at microtubule nucleation sites and is required for microtubule nucleation. J. Cell Sci. 115, 2423–2431 (2002).
Chan, J., Calder, G., Doonan, J. H. & Lloyd, C. W. Mobile microtubule nucleation sites revealed in Arabidopsis by EB1. EB1 reveals mobile microtubule nucleation sites in Arabidopsis. Nature Cell Biol. 5, 967–971 (2003). This work shows that cortical microtubules are nucleated from multiple foci that are labelled with the microtubule-end-binding protein, EB1.
Straube, A., Brill, M., Oakley, B. R., Horio, T. & Steinberg, G. Microtubule organization requires cell cycle-dependent nucleation at dispersed cytoplasmic sites: polar and perinuclear microtubule organizing centers in the plant pathogen Ustilago maydis. Mol. Biol. Cell 14, 642–657 (2003).
Rogers, S. L., Rogers, G. C., Sharp, D. J. & Vale, R. D. Drosophila EB1 is important for proper assembly, dynamics, and positioning of the mitotic spindle. J. Cell Biol. 158, 873–884 (2002).
McNally, F. J., Okawa, K., Iwamatsu, A. & Vale, R. D. Katanin, the microtubule-severing ATPase, is concentrated at centrosomes. J. Cell Sci. 109, 561–567 (1996).
McClinton, R. S., Chandler, J. S. & Callis, J. cDNA isolation, characterization, and protein intracellular localization of a katanin-like p60 subunit from Arabidopsis thaliana. Protoplasma 216, 181–190 (2001).
Stoppin-Mellet, V., Gaillard, J. & Vantard, M. Functional evidence for in vitro microtubule severing by the plant katanin homologue. Biochem. J. 365, 337–342 (2002).
Burk, D. H. & Ye, Z. H. Alteration of oriented deposition of cellulose microfibrils by mutation of a katanin-like microtubule-severing protein. Plant Cell 14, 2145–2160 (2002). Mutation of the microtubule-severing protein katanin is shown to perturb microtubule organization, which results in disorganization of cellulose in the cell wall.
Bouquin, T., Mattsson, O., Naested, H., Foster, R. & Mundy, J. The Arabidopsis lue1 mutant defines a katanin p60 ortholog involved in hormonal control of microtubule orientation during cell growth. J. Cell Sci. 116, 791–801 (2003).
Burk, D. H., Liu, B., Zhong, R., Morrison, W. H. & Ye, Z. H. A katanin-like protein regulates normal cell wall biosynthesis and cell elongation. Plant Cell 13, 807–827 (2001).
Bichet, A., Desnos, T., Turner, S., Grandjean, O. & Hofte, H. BOTERO1 is required for normal orientation of cortical microtubules and anisotropic cell expansion in Arabidopsis. Plant J. 25, 137–148 (2001).
Webb, M., Jouannic, S., Foreman, J., Linstead, P. & Dolan, L. Cell specification in the Arabidopsis root epidermis requires the activity of ECTOPIC ROOT HAIR 3 — a katanin-p60 protein. Development 129, 123–131 (2002).
Duckett, C. M. & Lloyd, C. W. Gibberellic acid-induced microtubule reorientation in dwarf peas is accompanied by rapid modification of an α-tubulin isotype. Plant J. 5, 363–372 (1994).
Reddy, A. S. & Day, I. S. Kinesins in the Arabidopsis genome: a comparative analysis among eukaryotes. BMC Genomics 2, 2 <http://www.biomedcentral.com/1471-2164/2/2> (2001).
Zhong, R., Burk, D. H., Morrison, W. H. & Ye, Z. H. A kinesin-like protein is essential for oriented deposition of cellulose microfibrils and cell wall strength. Plant Cell 14, 3101–3117 (2002).
Preuss, M. L., Delmer, D. P. & Liu, B. The cotton kinesin-like calmodulin-binding protein associates with cortical microtubules in cotton fibers. Plant Physiol. 132, 154–160 (2003).
Hush, J. M., Wadsworth, P., Callaham, D. A. & Hepler, P. K. Quantification of microtubule dynamics in living plant cells using fluorescence redistribution after photobleaching. J. Cell Sci. 107, 775–784 (1994).
Jiang, C. -J. & Sonobe, S. Identification and preliminary characterization of a 65kDa higher-plant microtubule-associated protein. J. Cell Sci. 105, 891–901 (1993).
Chan, J., Jensen, C. G., Jensen, L. C., Bush, M. & Lloyd, C. W. The 65-kDa carrot microtubule-associated protein forms regularly arranged filamentous cross-bridges between microtubules. Proc. Natl Acad. Sci. USA 96, 14931–14936 (1999).
Hussey, P. J., Hawkins, T. J., Igarashi, H., Kaloriti, D. & Smertenko, A. The plant cytoskeleton: recent advances in the study of the plant microtubule-associated proteins MAP-65, MAP-190 and the Xenopus MAP215-like protein, MOR1. Plant Mol. Biol. 50, 915–924 (2002).
Chan, J. et al. Identification of a MAP65 isoform involved in directional expansion of plant cells. FEBS Lett. 534, 161–163 (2003).
Yasuhara, H., Muraoka, M., Shogaki, H., Mori, H. & Sonobe, S. TMBP200, a microtubule bundling polypeptide isolated from telophase tobacco BY-2 cells is a MOR1 homologue. Plant Cell Physiol. 43, 595–603 (2002).
Whittington, A. T. et al. MOR1 is essential for organizing cortical microtubules in plants. Nature 411, 610–613 (2001).
Twell, D. et al. MOR1/GEM1 has an essential role in the plant-specific cytokinetic phragmoplast. Nature Cell Biol. 4, 711–714 (2002).
Hussey, P. J. & Hawkins, T. J. Plant microtubule-associated proteins: the HEAT is off in temperature-sensitive mor1. Trends Plant Sci. 6, 389–392 (2001).
Tournebize, R. et al. Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts. Nature Cell Biol. 2, 13–19 (2000).
Shirasu-Hiza, M., Coughlin, P. & Mitchison, T. Identification of XMAP215 as a microtubule-destabilizing factor in Xenopus egg extract by biochemical purification. J. Cell Biol. 161, 349–358 (2003).
van Breugel, M., Drechsel, D. & Hyman, A. Stu2p, the budding yeast member of the conserved Dis1/XMAP215 family of microtubule-associated proteins is a plus end-binding microtubule destabilizer. J. Cell Biol. 161, 359–369 (2003).
McNally, F. Microtubule dynamics: new surprises from an old MAP. Curr. Biol. 13, R597–R599 (2003).
Marc, J., Sharkey, D. E., Durso, N. A., Zhang, M. & Cyr, R. J. Isolation of a 90-kD microtubule-associated protein from Tobacco membranes. Plant Cell. 8, 2127–2138 (1996).
Gardiner, J. C. et al. A 90-kD phospholipase D from tobacco binds to microtubules and the plasma membrane. Plant Cell 13, 2143–2158 (2001).
Gardiner, J., Collings. D. A., Harper, J. D. & Marc, J. The effects of the phospholipase D-antagonist 1-butanol on seedling development and microtubule organisation in Arabidopsis. Plant Cell Physiol. 44,687–696 (2003).
Dhonukshe, P., Laxalt, A. M., Goedhart, J., Gadella, T. W. & Munnik, T. Phospholipase D activation correlates with microtubule reorganization in living plant cells. Plant Cell. 15, 2666–2679 (2003). References 44–47 show that a phospholipase D links cortical microtubules to the plasma membrane and suggest how signalling chains might alter cell morphology.
Baskin, T. I. On the alignment of cellulose microfibrils by cortical microtubules: a review and a model. Protoplasma 215, 150–171 (2001).
Arioli, T. et al. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 279, 717–720 (1998).
Sugimoto, K., Williamson, R. E. & Wasteneys, G. O. Wall architecture in the cellulose-deficient rsw1 mutant of Arabidopsis thaliana: microfibrils but not microtubules lose their transverse alignment before microfibrils become unrecognizable in the mitotic and elongation zones of roots. Protoplasma 215, 172–183 (2001).
Gardiner, J. C., Taylor, N. G. & Turner, S. R. Control of cellulose synthase complex localization in developing xylem. Plant Cell 15, 1740–1748 (2003).
Sugimoto, K., Himmelspach, R., Williamson, R. E. & Wasteneys, G. O. Mutation or drug-dependent microtubule disruption causes radial swelling without altering parallel cellulose microfibril deposition in Arabidopsis root cells. Plant Cell 15, 1414–1429 (2003). This work shows that the relationship between microtubules and cellulose alignment is far from simple.
Wiedemeier, A. M. et al. Mutant alleles of Arabidopsis RADIALLY SWOLLEN 4 and 7 reduce growth anisotropy without altering the transverse orientation of cortical microtubules or cellulose microfibrils. Development 129, 4821–4830 (2002).
McCartney, L., Steele-King, C. G., Jordan, E. & Knox, J. P. Cell wall pectic (1→4)-β-D-galactan marks the acceleration of cell elongation in the Arabidopsis seedling root meristem. Plant J. 33, 447–454 (2003).
Park, S. K., Howden, R. & Twell, D. The Arabidopsis thaliana gametophytic mutation gemini pollen1 disrupts microspore polarity, division asymmetry and pollen cell fate. Development 125, 3789–3799 (1998).
Mollinari, C. et al. PRC1 is a microtubule binding and bundling protein essential to maintain the mitotic spindle midzone. J. Cell Biol. 157, 1175–1186 (2002).
Acknowledgements
The authors' work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) by way of a grant-in-aid to the John Innes Centre.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- MICROTUBULE
-
A hollow tube, 25 nm in diameter, that is formed by the lateral association of (usually) 13 protofilaments, which are polymers of α- and β-tubulin subunits.
- EXTRACELLULAR MATRIX
-
(ECM). The external coating of a cell. The plant cell's ECM — the cell wall — is mainly composed of various classes of complex carbohydrate that are structured around the fibrous polysaccharide, cellulose, whereas the main fibrous element of the animal cell's ECM is the protein collagen.
- CENTROSOME
-
The main microtubule-organizing centre of animal cells.
- FIBROBLAST
-
A common cell type that is found in the connective tissue in many parts of the body. Fibroblasts secrete an extracellular matrix that is rich in collagen and other macromolecules, and that connects cell layers.
- CYTOPLASMIC STREAMING
-
The actomyosin-based movement of vesicles and organelles around the plant cell, on tracks that are provided by actin filaments.
- MICROTUBULE-ASSOCIATED PROTEIN
-
A protein that, in the loosest sense, binds to microtubules. More stringently, it is a protein that co-purifies with microtubules in vitro.
- INTERPHASE
-
The period between two mitotic divisions.
- YEAST TWO-HYBRID APPROACH
-
A technique that is used to test if two proteins physically interact with each other. One protein is fused to the GAL4 activation domain and the other to the GAL4 DNA-binding domain, and both fusion proteins are introduced into yeast. The expression of a GAL4-regulated reporter gene indicates that the two proteins physically interact.
- ORTHOLOGUES
-
A pair of genes, one in each species, that are descended from a single gene. If these two genes encode proteins with functional similarity they are referred to as functional orthologues.
- INFLORESCENCE STEM
-
The stem that carries the flowers.
- SECONDARY WALL
-
The flexible extracellular matrix that is deposited while the cell is still expanding is known as the primary cell wall. When expansion ceases, the secondary wall is laid down inside the primary wall, which makes it stronger.
- ANISOTROPIC
-
Unlike isotropic growth, which occurs in all directions, anisotropic growth has a preferred direction.
- KINESIN
-
A protein that uses the energy of ATP hydrolysis to move along a microtubule. Kinesins with the microtubule-binding domain at the amino-terminal head move cargo to the fast-growing plus end of the microtubule, whereas kinesins with motors at the carboxyl terminus move in the opposite direction.
- CELL PLATE
-
The flattened disk of immature cell wall that is deposited by the fusion of Golgi vesicles in the plane where the two half sets of phragmoplast microtubules overlap. It contains a polysaccharide, callose, which is composed of chains of β-1,3-linked glucosyl residues.
- HeLa CELLS
-
An established tissue-culture strain of human epidermoid carcinoma cells, which contain 70–80 chromosomes per cell. These cells were originally derived from tissue taken from a patient named Henrietta Lacks in 1951.
- PHOTOBLEACHING
-
The irreversible destruction, by any one of several different mechanisms, of a fluorophore that is under illumination.
- TAXOL
-
An antitumour agent that enhances the polymerization of tubulin and the subsequent stabilization of microtubules, thereby inhibiting mitosis and blocking the cell cycle.
- CLADE
-
A taxon or other grouping of organisms consisting of a single species and its descendents.
- TELOPHASE
-
The final stage of mitosis or meiosis in which the nuclei form in the daughter cells.
Rights and permissions
About this article
Cite this article
Lloyd, C., Chan, J. Microtubules and the shape of plants to come. Nat Rev Mol Cell Biol 5, 13–23 (2004). https://doi.org/10.1038/nrm1277
Issue Date:
DOI: https://doi.org/10.1038/nrm1277
This article is cited by
-
The role of microtubules in microalgae: promotion of lipid accumulation and extraction
Biotechnology for Biofuels and Bioproducts (2023)
-
CLASP balances two competing cell division plane cues during leaf development
Nature Plants (2022)
-
Microtubules play a crucial role in regulating actin organization and cell initiation in cotton fibers
Plant Cell Reports (2022)
-
OsFH15, a class I formin, interacts with microfilaments and microtubules to regulate grain size via affecting cell expansion in rice
Scientific Reports (2017)
-
Characterization of the GRAS transcription factor SCARECROW-LIKE 28’s role in Arabidopsis root growth
Journal of Plant Biology (2017)