Key Points
-
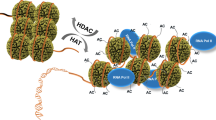
In the yeast Saccharomyces cerevisiae, histone acetylation and deacetylation occur through both targeted and global mechanisms. In the targeted mechanism, histone acetyltransferase (HAT) and deacetylase (HDAC) enzymes are recruited to promoters by specific transcriptional activators and repressors, respectively. In the global mechanism, HATs and HDACs enzymatically modify histones throughout large chromatin domains, including coding sequences, without any apparent DNA-sequence specificity.
-
Each of five related HDACs in yeast (Hda1, Rpd3, Hos1, Hos2 and Hos3) deacetylates specific sets of genes and regions of the genome. Such 'division of labour' might organize the affected genes in functional and/or physical domains, such as the HAST (for Hda1-affected subtelomeric) domains that are deacetylated by Hda1 and contain genes that are required for growth in adverse conditions.
-
In addition to their effects on chromatin structure, specific acetylated and deacetylated lysine residues in histones function as binding sites for factors that interact with chromatin. A bromodomain is a protein motif that binds to singly or doubly acetylated histone amino termini. It is found in several eukaryotic transcription factors, as well as HATs and members of chromatin-remodelling complexes.
-
Other than transcription, histone acetylation and deacetylation function in the regulation of other DNA-based processes. In DNA replication, increased histone acetylation directly causes the earlier firing of replication origins in yeast. In DNA repair, a single acetylated lysine in histone H4 is required for the proper repair of double-strand breaks. In heterochromatin, unacetylated lysine 16 of histone H4 is required for the formation of telomeric heterochromatin, whereas acetylation of this lysine functions as a barrier to the spread of heterochromatin.
-
Contrary to the repressive role of other known deacetylases, the Hos2 HDAC binds to and deacetylates histones in the coding region of genes only during gene activity and is required for efficient transcriptional activation.
Abstract
Histone acetylation and deacetylation in the yeast Saccharomyces cerevisiae occur by targeting acetyltransferase and deacetylase enzymes to gene promoters and, in an untargeted and global manner, by affecting most nucleosomes. Recently, new roles for histone acetylation have been uncovered, not only in transcription but also in DNA replication, repair and heterochromatin formation. Interestingly, specific acetylatable lysines can function as binding sites for regulatory factors. Moreover, histone deacetylation is not only repressive but can be required for gene activity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Allfrey, V. G., Faulkner, R. M. & Mirsky, A. E. Acetylation and methylation of histones and their possible role in the regulation of RNA synthesis. Proc. Natl Acad. Sci. USA 51, 786–794 (1964).
Lorch, Y., LaPointe, J. W. & Kornberg, R. D. Nucleosomes inhibit the initiation of transcription but allow chain elongation with the displacement of histones. Cell 49, 203–210 (1987).
Han, M. & Grunstein, M. Nucleosome loss activates yeast downstream promoters in vivo. Cell 55, 1137–1145 (1988).
Clark-Adams, C. D., Norris, D., Osley, M. A., Fassler, J. S. & Winston, F. Changes in histone gene dosage alter transcription in yeast. Genes Dev. 2, 150–159 (1988).
Durrin, L. K., Mann, R. K., Kayne, P. S. & Grunstein, M. Yeast histone H4 N-terminal sequence is required for promoter activation in vivo. Cell 65, 1023–1031 (1991).
Kuo, M. H. & Allis, C. D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays 20, 615–626 (1998).
Rundlett, S. E. et al. HDA1 and RPD3 are members of distinct yeast histone deacetylase complexes that regulate silencing and transcription. Proc. Natl Acad. Sci. USA 93, 14503–14508 (1996).
Taunton, J., Hassig, C. A. & Schreiber, S. L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rpd3p. Science 272, 408–411 (1996).
Zhang, Y. et al. SAP30, a novel protein conserved between human and yeast, is a component of a histone deacetylase complex. Mol. Cell 1, 1021–1031 (1998).
Lechner, T. et al. Sds3 (suppressor of defective silencing 3) is an integral component of the yeast Sin3·Rpd3 histone deacetylase complex and is required for histone deacetylase activity. J. Biol. Chem. 275, 40961–40966 (2000).
Loewith, R. et al. Pho23 is associated with the Rpd3 histone deacetylase and is required for its normal function in regulation of gene expression and silencing in Saccharomyces cerevisiae. J. Biol. Chem. 276, 24068–24074 (2001).
Kurdistani, S. K., Robyr, D., Tavazoie, S. & Grunstein, M. Genome-wide binding map of the histone deacetylase Rpd3 in yeast. Nature Genet. 31, 248–254 (2002). In this study a novel crosslinking protocol was used for chromatin immunoprecipitation of Rpd3.
Gavin, A. C. et al. Functional organization of the yeast proteome by systematic analysis of protein complexes. Nature 415, 141–147 (2002). This reference provides a searchable database for yeast protein–protein interactions.
Kadosh, D. & Struhl, K. Repression by Ume6 involves recruitment of a complex containing Sin3 corepressor and Rpd3 histone deacetylase to target promoters. Cell 89, 365–371 (1997). This study elucidates the promoter targeting mechanism of a yeast histone deacetylase.
Rundlett, S. E., Carmen, A. A., Suka, N., Turner, B. M. & Grunstein, M. Transcriptional repression by UME6 involves deacetylation of lysine 5 of histone H4 by RPD3. Nature 392, 831–835 (1998).
Kadosh, D. & Struhl, K. Targeted recruitment of the Sin3–Rpd3 histone deacetylase complex generates a highly localized domain of repressed chromatin in vivo. Mol. Cell. Biol. 18, 5121–5127 (1998).
Suka, N., Suka, Y., Carmen, A. A., Wu, J. & Grunstein, M. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol. Cell 8, 473–479 (2001). This study reports highly specific antibodies against individual sites of histone acetylation in yeast.
Deckert, J. & Struhl, K. Targeted recruitment of Rpd3 histone deacetylase represses transcription by inhibiting recruitment of Swi/Snf, SAGA, and TATA binding protein. Mol. Cell. Biol. 22, 6458–6470 (2002).
Wu, J., Suka, N., Carlson, M. & Grunstein, M. TUP1 utilizes histone H3/H2B-specific HDA1 deacetylase to repress gene activity in yeast. Mol. Cell 7, 117–126 (2001).
Kuo, M. H., vom Baur, E., Struhl, K. & Allis, C. D. Gcn4 activator targets Gcn5 histone acetyltransferase to specific promoters independently of transcription. Mol. Cell 6, 1309–1320 (2000).
Allard, S. et al. NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J. 18, 5108–5119 (1999).
Reid, J. L., Iyer, V. R., Brown, P. O. & Struhl, K. Coordinate regulation of yeast ribosomal protein genes is associated with targeted recruitment of Esa1 histone acetylase. Mol. Cell 6, 1297–1307 (2000).
Bernstein, B. E., Tong, J. K. & Schreiber, S. L. Genomewide studies of histone deacetylase function in yeast. Proc. Natl Acad. Sci. USA 97, 13708–13713 (2000).
Hughes, T. R. et al. Functional discovery via a compendium of expression profiles. Cell 102, 109–126 (2000). This paper provides a searchable database for the genome wide expression analysis of ∼300 conditions in yeast.
Robyr, D. et al. Microarray deacetylation maps determine genome-wide functions for yeast histone deacetylases. Cell 109, 437–446 (2002).
Wang, A., Kurdistani, S. & Grunstein, M. Requirement of Hos2 histone deacetylase for gene activity in yeast. Science 298, 1412–1414 (2002). This paper shows that a histone deacetylase is directly required for gene activity.
Holstege, F. C. et al. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95, 717–728 (1998).
Mutskov, V. et al. Persistent interactions of core histone tails with nucleosomal DNA following acetylation and transcription factor binding. Mol. Cell. Biol. 18, 6293–6304 (1998).
Tse, C., Sera, T., Wolffe, A. P. & Hansen, J. C. Disruption of higher-order folding by core histone acetylation dramatically enhances transcription of nucleosomal arrays by RNA polymerase III. Mol. Cell. Biol. 18, 4629–4638 (1998).
Horn, P. J. & Peterson, C. L. Molecular biology. Chromatin higher order folding — wrapping up transcription. Science 297, 1824–1827 (2002).
Hecht, A., Laroche, T., Strahl-Bolsinger, S., Gasser, S. M. & Grunstein, M. Histone H3 and H4 N-termini interact with SIR3 and SIR4 proteins: a molecular model for the formation of heterochromatin in yeast. Cell 80, 583–592 (1995).
Johnson, L. M., Kayne, P. S., Kahn, E. S. & Grunstein, M. Genetic evidence for an interaction between SIR3 and histone H4 in the repression of the silent mating loci in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 87, 6286–6290 (1990).
Hecht, A., Strahl-Bolsinger, S. & Grunstein, M. Spreading of transcriptional repressor SIR3 from telomeric heterochromatin. Nature 383, 92–96 (1996).
Carmen, A. A., Milne, L. & Grunstein, M. Acetylation of the yeast histone H4 N terminus regulates its binding to heterochromatin protein SIR3. J. Biol. Chem. 277, 4778–4781 (2002).
Braunstein, M., Sobel, R. E., Allis, C. D., Turner, B. M. & Broach, J. R. Efficient transcriptional silencing in Saccharomyces cerevisiae requires a heterochromatin histone acetylation pattern. Mol. Cell. Biol. 16, 4349–4356 (1996).
Megee, P. C., Morgan, B. A., Mittman, B. A. & Smith, M. M. Genetic analysis of histone H4: essential role of lysines subject to reversible acetylation. Science 247, 841–845 (1990).
Tamkun, J. W. et al. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell 68, 561–572 (1992).
Winston, F. & Allis, C. D. The bromodomain: a chromatin-targeting module? Nature Struct. Biol. 6, 601–604 (1999).
Dhalluin, C. et al. Structure and ligand of a histone acetyltransferase bromodomain. Nature 399, 491–496 (1999).
Jacobson, R. H., Ladurner, A. G., King, D. S. & Tjian, R. Structure and function of a human TAFII250 double bromodomain module. Science 288, 1422–1425 (2000).
Agalioti, T., Chen, G. & Thanos, D. Deciphering the transcriptional histone acetylation code for a human gene. Cell 111, 381–392 (2002). This report provides details on the recognition of specifically acetylated lysines in histones H3 and H4 by two transcription factors.
Syntichaki, P., Topalidou, I. & Thireos, G. The Gcn5 bromodomain co-ordinates nucleosome remodelling. Nature 404, 414–417 (2000).
Hassan, A. H., Neely, K. E. & Workman, J. L. Histone acetyltransferase complexes stabilize swi/snf binding to promoter nucleosomes. Cell 104, 817–827 (2001).
Hassan, A. H. et al. Function and selectivity of bromodomains in anchoring chromatin-modifying complexes to promoter nucleosomes. Cell 111, 369–379 (2002). References 39–44 report data on the structure and function of bromodomains.
Vogelauer, M., Wu, J., Suka, N. & Grunstein, M. Global histone acetylation and deacetylation in yeast. Nature 408, 495–498 (2000).
Krebs, J. E., Fry, C. J., Samuels, M. L. & Peterson, C. L. Global role for chromatin remodeling enzymes in mitotic gene expression. Cell 102, 587–598 (2000).
Waterborg, J. H. Dynamics of histone acetylation in Saccharomyces cerevisiae. Biochemistry 40, 2599–2605 (2001).
Yamagoe, S. et al. Interaction of histone acetylases and deacetylases in vivo. Mol. Cell. Biol. 23, 1025–1033 (2003).
Katan-Khaykovich, Y. & Struhl, K. Dynamics of global histone acetylation and deacetylation in vivo: rapid restoration of normal histone acetylation status upon removal of activators and repressors. Genes. Dev. 16, 743–752 (2002).
Ivessa, A. S. & Zakian, V. A. To fire or not to fire: origin activation in Saccharomyces cerevisiae ribosomal DNA. Genes Dev. 16, 2459–2464 (2002).
Ferguson, B. M. & Fangman, W. L. A position effect on the time of replication origin activation in yeast. Cell 68, 333–339 (1992).
Ferguson, B. M., Brewer, B. J., Reynolds, A. E. & Fangman, W. L. A yeast origin of replication is activated late in S phase. Cell 65, 507–515 (1991).
Friedman, K. L. et al. Multiple determinants controlling activation of yeast replication origins late in S phase. Genes Dev. 10, 1595–1607 (1996).
Stevenson, J. B. & Gottschling, D. E. Telomeric chromatin modulates replication timing near chromosome ends. Genes Dev. 13, 146–151 (1999).
Lipford, J. R. & Bell, S. P. Nucleosomes positioned by ORC facilitate the initiation of DNA replication. Mol. Cell 7, 21–30 (2001).
Vogelauer, M., Rubbi, L., Lucas, I., Brewer, B. J. & Grunstein, M. Histone acetylation regulates the time of replication origin firing. Mol. Cell 10, 1223–1233 (2002).
Cimbora, D. M. et al. Long-distance control of origin choice and replication timing in the human β-globin locus are independent of the locus control region. Mol. Cell. Biol. 20, 5581–5591 (2000).
Iizuka, M. & Stillman, B. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J. Biol. Chem. 274, 23027–23034 (1999).
Burke, T. W., Cook, J. G., Asano, M. & Nevins, J. R. Replication factors MCM2 and ORC1 interact with the histone acetyltransferase HBO1. J. Biol. Chem. 276, 15397–15408 (2001).
Megee, P. C., Morgan, B. A. & Smith, M. M. Histone H4 and the maintenance of genome integrity. Genes Dev. 9, 1716–1727 (1995).
Bird, A. W. et al. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 419, 411–415 (2002). This paper reports the direct role of histone acetylation in DNA repair.
Choy, J. S. & Kron, S. J. NuA4 subunit Yng2 function in intra-S-phase DNA damage response. Mol. Cell. Biol. 22, 8215–8225 (2002).
Qin, S. & Parthun, M. R. Histone h3 and the histone acetyltransferase hat1p contribute to DNA double-strand break repair. Mol. Cell. Biol. 22, 8353–8365 (2002).
Koyama, H., Itoh, M., Miyahara, K. & Tsuchiya, E. Abundance of the RSC nucleosome-remodeling complex is important for the cells to tolerate DNA damage in Saccharomyces cerevisiae. FEBS Lett. 531, 215–221 (2002).
Sobel, R. E., Cook, R. G., Perry, C. A., Annunziato, A. T. & Allis, C. D. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc. Natl Acad. Sci. USA 92, 1237–1241 (1995).
Moretti, P., Freeman, K., Coodly, L. & Shore, D. Evidence that a complex of SIR proteins interacts with the silencer and telomere-binding protein RAP1. Genes Dev. 8, 2257–2269 (1994).
Luo, K., Vega-Palas, M. A. & Grunstein, M. Rap1–Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev. 16, 1528–1539 (2002).
Hoppe, G. J. et al. Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol. Cell. Biol. 22, 4167–4180 (2002).
Rusche, L. N., Kirchmaier, A. L. & Rine, J. Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 2207–2222 (2002).
Kayne, P. S. et al. Extremely conserved histone H4 N terminus is dispensable for growth but essential for repressing the silent mating loci in yeast. Cell 55, 27–39 (1988).
Thompson, J. S., Ling, X. & Grunstein, M. Histone H3 amino terminus is required for telomeric and silent mating locus repression in yeast. Nature 369, 245–247 (1994).
Renauld, H. et al. Silent domains are assembled continuously from the telomere and are defined by promoter distance and strength, and by SIR3 dosage. Genes Dev. 7, 1133–1145 (1993).
Donze, D. & Kamakaka, R. T. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. EMBO J. 20, 520–531 (2001).
Suka, N., Luo, K. & Grunstein, M. Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nature Genet. 32, 378–383 (2002).
Kimura, A., Umehara, T. & Horikoshi, M. Chromosomal gradient of histone acetylation established by Sas2p and Sir2p functions as a shield against gene silencing. Nature Genet. 32, 370–377 (2002).
Orphanides, G. & Reinberg, D. RNA polymerase II elongation through chromatin. Nature 407, 471–475 (2000).
Protacio, R. U., Li, G., Lowary, P. T. & Widom, J. Effects of histone tail domains on the rate of transcriptional elongation through a nucleosome. Mol. Cell. Biol. 20, 8866–8878 (2000).
Otero, G. et al. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol. Cell 3, 109–118 (1999).
Wittschieben, B. O. et al. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol. Cell 4, 123–128 (1999).
Pokholok, D. K., Hannett, N. M. & Young, R. A. Exchange of RNA polymerase II initiation and elongation factors during gene expression in vivo. Mol. Cell 9, 799–809 (2002).
Logie, C., Tse, C., Hansen, J. C. & Peterson, C. L. The core histone N-terminal domains are required for multiple rounds of catalytic chromatin remodeling by the SWI/SNF and RSC complexes. Biochemistry 38, 2514–2522 (1999).
Kim, Y. & Clark, D. J. SWI/SNF-dependent long-range remodeling of yeast HIS3 chromatin. Proc. Natl Acad. Sci. USA 99, 15381–15386 (2002).
Lo, W. S. et al. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol. Cell 5, 917–926 (2000).
Sun, Z. W. & Allis, C. D. Ubiquitination of histone H2B regulates H3 methylation and gene silencing in yeast. Nature 418, 104–108 (2002).
Ng, H. H., Xu, R. M., Zhang, Y. & Struhl, K. Ubiquitination of histone H2B by Rad6 is required for efficient Dot1-mediated methylation of histone H3 lysine 79. J. Biol. Chem. 277, 34655–34657 (2002). References 84 and 85 describe evidence that modification of one histone affects modifications of other histones.
Santos-Rosa, H. et al. Active genes are tri-methylated at K4 of histone H3. Nature 419, 407–411 (2002).
Bannister, A. J. et al. Selective recognition of methylated lysine 9 on histone H3 by the HP1 chromo domain. Nature 410, 120–124 (2001).
Noma, K., Allis, C. D. & Grewal, S. I. Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science 293, 1150–1155 (2001).
Strahl, B. D. & Allis, C. D. The language of covalent histone modifications. Nature 403, 41–45 (2000).
Turner, B. M. & O'Neill, L. P. Histone acetylation in chromatin and chromosomes. Semin. Cell. Biol. 6, 229–236 (1995).
Schreiber, S. L. & Bernstein, B. E. Signaling network model of chromatin. Cell 111, 771–778 (2002). References 89–91 provide alternative interpretations of the complexity of histone modifications.
Acknowledgements
S.K.K. is a Howard Hughes Medical Institute Physician Postdoctoral Fellow. This work was supported by Public Health Service grants from the National Institutes of Health to M.G.
Author information
Authors and Affiliations
Corresponding author
Glossary
- ACETYLATION
-
The covalent addition of the acetyl chemical group to lysine residues in histones and other proteins that neutralizes the positive charge of the ε-amino group of the lysine side chain. Deacetylation removes the acetyl group, thereby restoring the positive charge.
- NUCLEOSOME
-
The fundamental unit of chromatin, which is composed of 147 bp of DNA wrapped around an octameric core of histone proteins (two molecules each of H2A, H2B, H3 and H4).
- HISTONE ACETYLTRANSFERASE
-
(HAT). An enzyme that catalyses the addition of acetyl groups to the lysine residues of histones.
- HISTONE DEACETYLASE
-
(HDAC). An enzyme that catalyses the removal of acetyl groups from the lysine residues of histones.
- HETEROCHROMATIN
-
A region of chromosome that is structurally condensed or inaccessible to molecular probes in interphase, and is generally transcriptionally silent and late replicating in S phase.
- CHROMATIN IMMUNOPRECIPITATION
-
(ChIP). A technique that combines immunoprecipitation of chromatin fragments and polymerase chain reaction (PCR) to semi-quantifiably map sites of protein–DNA interaction in vivo.
- CHROMATIN REMODELLING
-
An alteration in chromatin structure that is often achieved by the covalent modifications of histones and/or the action of ATP-dependent remodelling complexes. Remodelling can allow the increased access of transcription factors to chromatin.
- BASAL TRANSCRIPTION
-
Transcription that occurs in the absence of any activating signal.
- DNA MICROARRAY
-
A high-throughput screening method developed initially for genome-wide differential expression analysis. It involves probing known DNA sequences that are printed at high density on a glass slide.
- EUCHROMATIN
-
Regions of the genome that are less condensed in interphase and contain most active genes.
- ENHANCER
-
A regulatory DNA sequence that can influence the rate of transcription of a promoter at a distance.
- SILENT MATING-TYPE LOCI
-
Two regions on yeast chromosome III that determine the mating type of a yeast cell as a or α. They are assembled into heterochromatin by proteins (for example, Rap1, Sir2–4) that also participate in the formation of telomeric heterochromatin.
Rights and permissions
About this article
Cite this article
Kurdistani, S., Grunstein, M. Histone acetylation and deacetylation in yeast. Nat Rev Mol Cell Biol 4, 276–284 (2003). https://doi.org/10.1038/nrm1075
Issue Date:
DOI: https://doi.org/10.1038/nrm1075
This article is cited by
-
Genomic disturbance of vitellogenin 2 (vtg2) leads to vitellin membrane deficiencies and significant mortalities at early stages of embryonic development in zebrafish (Danio rerio)
Scientific Reports (2023)
-
Transcriptomic analysis reveals hub genes and pathways in response to acetic acid stress in Kluyveromyces marxianus during high-temperature ethanol fermentation
Stress Biology (2023)
-
The histone deacetylase HOS2 controls pathogenicity through regulation of melanin biosynthesis and appressorium formation in Colletotrichum gloeosporioides
Phytopathology Research (2022)
-
The histone deacetylase Cfhos2 is a key epigenetic factor regulating appressorium development and pathogenesis in apple Glomerella leaf spot fungus Colletotrichum fructicola
Phytopathology Research (2022)
-
Histone Deacetylases in Rice Development and Stress Responses
Journal of Plant Biology (2022)