Key Points
-
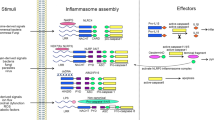
Inflammasomes are multiprotein complexes that assemble upon the sensing of danger signals and initiate the release of proinflammatory cytokines IL-1β and IL-18 via caspase-1 activation
-
By responding to low-threshold signals, the inflammasome is able to fine-tune the inflammatory response
-
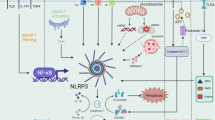
The balance between a healthy inflammatory response and chronic damage is delicate and several inflammasomes, such as NLRP3, NLRP6 and AIM2, also have a role in liver diseases
-
Among other diseases, inflammasome activation contributes to alcoholic steatohepatitis and NASH, chronic HCV infection, ischaemia–reperfusion injury, paracetamol-induced liver injury and liver fibrosis
Abstract
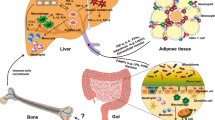
Inflammation contributes to the pathogenesis of most acute and chronic liver diseases. Inflammasomes are multiprotein complexes that can sense danger signals from damaged cells and pathogens and assemble to mediate caspase-1 activation, which proteolytically activates the cytokines IL-1β and IL-18. In contrast to other inflammatory responses, inflammasome activation uniquely requires two signals to induce inflammation, therefore setting an increased threshold. IL-1β, generated upon caspase-1 activation, provides positive feed-forward stimulation for inflammatory cytokines, thereby amplifying inflammation. Inflammasome activation has been studied in different human and experimental liver diseases and has been identified as a major contributor to hepatocyte damage, immune cell activation and amplification of liver inflammation. In this Review, we discuss the different types of inflammasomes, their activation and biological functions in the context of liver injury and disease progression. Specifically, we focus on the triggers of inflammasome activation in alcoholic steatohepatitis and NASH, chronic HCV infection, ischaemia–reperfusion injury and paracetamol-induced liver injury. The application and translation of these discoveries into therapies promises novel approaches in the treatment of inflammation in liver disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Janeway, C. A., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 54, 1–13 (1989).
Matzinger, P. Tolerance, danger, and the extended family. Annu. Rev. Immunol. 12, 991–1045 (1994).
Shi, Y. Caught red-handed: uric acid is an agent of inflammation. J. Clin. Invest. 120, 1809–1811 (2010).
Seki, E. & Brenner, D. A. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 48, 322–335 (2008).
Chassaing, B., Etienne-Mesmin, L. & Gewirtz, A. T. Microbiota-liver axis in hepatic disease. Hepatology 59, 328–339 (2014).
Schwabe, R. F., Seki, E. & Brenner, D. A. Toll-like receptor signaling in the liver. Gastroenterology 130, 1886–1900 (2006).
Gao, B. et al. Innate immunity in alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G516–G525 (2011).
Szabo, G., Dolganiuc, A. & Mandrekar, P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology 44, 287–298 (2006).
Kubes, P. & Mehal, W. Z. Sterile inflammation in the liver. Gastroenterology 143, 1158–1172 (2012).
Chen, G. Y. & Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 10, 826–837 (2010).
Warren, S. E. et al. Cutting edge: cytosolic bacterial DNA activates the inflammasome via Aim2. J. Immunol. 185, 818–821 (2010).
Ogura, Y., Sutterwala, F. S. & Flavell, R. A. The inflammasome: first line of the immune response to cell stress. Cell 126, 659–662 (2006).
Szabo, G. & Csak, T. Inflammasomes in liver diseases. J. Hepatol. 57, 642–654 (2012).
Martinon, F., Burns, K. & Tschopp, J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 (2002).
Schroder, K. & Tschopp, J. The inflammasomes. Cell 140, 821–832 (2010).
Kumar, H., Kawai, T. & Akira, S. Pathogen recognition by the innate immune system. Int. Rev. Immunol. 30, 16–34 (2011).
Mandrekar, P., Ambade, A., Lim, A., Szabo, G. & Catalano, D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 54, 2185–2197 (2011).
Petrasek, J. et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 122, 3476–3489 (2012).
Miura, K. et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1β in mice. Gastroenterology 139, 323–334 (2010).
Mariathasan, S. et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 (2006).
Rock, K. L., Kataoka, H. & Lai, J. J. Uric acid as a danger signal in gout and its comorbidities. Nat. Rev. Rheumatol. 9, 13–23 (2013).
Csak, T. et al. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 54, 133–144 (2011).
Matsuzaka, T. et al. Elovl6 promotes nonalcoholic steatohepatitis. Hepatology 56, 2199–2208 (2012).
Wen, H. et al. Fatty acid-induced NLRP3-ASC inflammasome activation interferes with insulin signaling. Nat. Immunol. 12, 408–415 (2011).
Vandanmagsar, B. et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 17, 179–188 (2011).
Shulga, N. & Pastorino, J. G. Hexokinase II binding to mitochondria is necessary for Kupffer cell activation and is potentiated by ethanol exposure. J. Biol. Chem. 289, 26213–26225 (2014).
Petrasek, J., Dolganiuc, A., Csak, T., Kurt-Jones, E. A. & Szabo, G. Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology 140, 697–708.e4 (2011).
Granowitz, E. V., Vannier, E., Poutsiaka, D. D. & Dinarello, C. A. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: II. IL-1 receptor antagonist inhibits lipopolysaccharide-induced cytokine synthesis by human monocytes. Blood 79, 2364–2369 (1992).
Granowitz, E. V., Clark, B. D., Vannier, E., Callahan, M. V. & Dinarello, C. A. Effect of interleukin-1 (IL-1) blockade on cytokine synthesis: I. IL-1 receptor antagonist inhibits IL-1-induced cytokine synthesis and blocks the binding of IL-1 to its type II receptor on human monocytes. Blood 79, 2356–2363 (1992).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Mehal, W. & Imaeda, A. Cell death and fibrogenesis. Semin. Liver Dis. 30, 226–231 (2010).
Cosgrove, B. D. et al. An inducible autocrine cascade regulates rat hepatocyte proliferation and apoptosis responses to tumor necrosis factor-α. Hepatology 48, 276–288 (2008).
Dinarello, C. A. The role of the interleukin-1-receptor antagonist in blocking inflammation mediated by interleukin-1. N. Engl. J. Med. 343, 732–734 (2000).
Gross, O., Thomas, C. J., Guarda, G. & Tschopp, J. The inflammasome: an integrated view. Immunol. Rev. 243, 136–151 (2011).
Davis, B. K., Wen, H. & Ting, J. P. The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735 (2011).
Guma, M. et al. Caspase 1-independent activation of interleukin-1β in neutrophil-predominant inflammation. Arthritis Rheum. 60, 3642–3650 (2009).
Joosten, L. A. et al. Inflammatory arthritis in caspase 1 gene-deficient mice: contribution of proteinase 3 to caspase 1-independent production of bioactive interleukin-1β. Arthritis Rheum. 60, 3651–3662 (2009).
Greten, F. R. et al. NF-κB is a negative regulator of IL-1β secretion as revealed by genetic and pharmacological inhibition of IKKβ. Cell 130, 918–931 (2007).
Mayer-Barber, K. D. et al. Caspase-1 independent IL-1β production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J. Immunol. 184, 3326–3330 (2010).
Fantuzzi, G. et al. Response to local inflammation of IL-1 beta-converting enzyme- deficient mice. J. Immunol. 158, 1818–1824 (1997).
Coeshott, C. et al. Converting enzyme-independent release of tumor necrosis factor α and IL-1β from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc. Natl Acad. Sci. USA 96, 6261–6266 (1999).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Dinarello, C. A. Interleukin-18, a proinflammatory cytokine. Eur. Cytokine Netw. 11, 483–486 (2000).
Henao-Mejia, J. et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 (2012).
Imaeda, A. B. et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J. Clin. Invest. 119, 305–314 (2009).
Serti, E. et al. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology 147, 209–220 (2014).
Arshad, M. I., Piquet-Pellorce, C. & Samson, M. IL-33 and HMGB1 alarmins: sensors of cellular death and their involvement in liver pathology. Liver Int. 32, 1200–1210 (2012).
Villarreal, D. O. & Weiner, D. B. Interleukin 33: a switch-hitting cytokine. Curr. Opin. Immunol. 28, 102–106 (2014).
Carriere, V. et al. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc. Natl Acad. Sci. USA 104, 282–287 (2007).
Schmitz, J. et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity 23, 479–490 (2005).
Cayrol, C. & Girard, J. P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl Acad. Sci. USA 106, 9021–9026 (2009).
Luzina, I. G. et al. Full-length IL-33 promotes inflammation but not Th2 response in vivo in an ST2-independent fashion. J. Immunol. 189, 403–410 (2012).
Volarevic, V. et al. Protective role of IL-33/ST2 axis in Con A-induced hepatitis. J. Hepatol. 56, 26–33 (2012).
Marvie, P. et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J. Cell. Mol. Med. 14, 1726–1739 (2010).
Yin, H. et al. Pretreatment with soluble ST2 reduces warm hepatic ischemia/reperfusion injury. Biochem. Biophys. Res. Commun. 351, 940–946 (2006).
Amatucci, A. et al. Recombinant ST2 boosts hepatic Th2 response in vivo. J. Leukoc. Biol. 82, 124–132 (2007).
Oboki, K. et al. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl Acad. Sci. USA 107, 18581–18586 (2010).
Schroder, K., Zhou, R. & Tschopp, J. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327, 296–300 (2010).
Csak, T. et al. Both bone marrow-derived and non-bone marrow-derived cells contribute to AIM2 and NLRP3 inflammasome activation in a MyD88-dependent manner in dietary steatohepatitis. Liver Int. 34, 1402–1413 (2014).
Bauernfeind, F. G. et al. Cutting edge: NF-κB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J. Immunol. 183, 787–791 (2009).
Martinon, F., Petrilli, V., Mayor, A., Tardivel, A. & Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 (2006).
Iyer, S. S. et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl Acad. Sci. USA 106, 20388–20393 (2009).
Latz, E. The inflammasomes: mechanisms of activation and function. Curr. Opin. Immunol. 22, 28–33 (2010).
Ioannou, G. N. et al. Cholesterol-lowering drugs cause dissolution of cholesterol crystals and disperse Kupffer cell crown-like structures during resolution of NASH. J. Lipid Res. 56, 277–285 (2014).
Halle, A. et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat. Immunol. 9, 857–865 (2008).
Zhou, R., Yazdi, A. S., Menu, P. & Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 469, 221–225 (2011).
Kim, H. Y., Kim, S. J. & Lee, S. M. Activation of NLRP3 and AIM2 inflammasomes in Kupffer cells in hepatic ischemia/reperfusion. FEBS J. 282, 259–270 (2014).
Muruve, D. A. et al. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 (2008).
Hornung, V. et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature 458, 514–518 (2009).
Nakahira, K. et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat. Immunol. 12, 222–230 (2011).
Rathinam, V. A. et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 (2010).
Poeck, H. et al. Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 β production. Nat. Immunol. 11, 63–69 (2010).
Lozano-Ruiz, B. et al. Absent in melanoma 2 triggers a heightened inflammasome response in ascitic fluid macrophages of patients with cirrhosis. J. Hepatol. 62, 64–71 (2015).
Grenier, J. M. et al. Functional screening of five PYPAF family members identifies PYPAF5 as a novel regulator of NF-kappaB and caspase-1. FEBS Lett. 530, 73–78 (2002).
Anand, P. K. et al. NLRP6 negatively regulates innate immunity and host defence against bacterial pathogens. Nature 488, 389–393 (2012).
Chen, G. Y., Liu, M., Wang, F., Bertin, J. & Nunez, G. A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J. Immunol. 186, 7187–7194 (2011).
Elinav, E. et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 (2011).
Normand, S. et al. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl Acad. Sci. USA 108, 9601–9606 (2011).
Chen, G. Y. Role of Nlrp6 and Nlrp12 in the maintenance of intestinal homeostasis. Eur. J. Immunol. 44, 321–327 (2014).
Wlodarska, M. et al. NLRP6 inflammasome orchestrates the colonic host-microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014).
O'Shea, R. S., Dasarathy, S., McCullough, A. J., Practice Guideline Committee of the American Association for the Study of Liver, D. & Practice Parameters Committee of the American College of, G. Alcoholic liver disease. Hepatology 51, 307–328 (2010).
Mandrekar, P. & Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 50, 1258–1266 (2009).
Peng, Y., French, B. A., Tillman, B., Morgan, T. R. & French, S. W. The inflammasome in alcoholic hepatitis: Its relationship with Mallory-Denk body formation. Exp. Mol. Pathol. 97, 305–313 (2014).
McClain, C. J. et al. Serum interleukin-1 (IL-1) activity in alcoholic hepatitis. Life Sci. 39, 1479–1485 (1986).
Gao, B. & Bataller, R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141, 1572–1585 (2011).
Xiao, J. et al. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int. J. Biol. Macromol. 69, 73–78 (2014).
Clemens, D. L. Use of cultured cells to study alcohol metabolism. Alcohol Res. Health 29, 291–295 (2006).
Szabo, G. Gut–liver axis in alcoholic liver disease. Gastroenterology 148, 30–36 (2015).
Inokuchi, S. et al. Toll-like receptor 4 mediates alcohol-induced steatohepatitis through bone marrow-derived and endogenous liver cells in mice. Alcohol Clin. Exp. Res. 35, 1509–1518 (2011).
Hoek, J. B., Cahill, A. & Pastorino, J. G. Alcohol and mitochondria: a dysfunctional relationship. Gastroenterology 122, 2049–2063 (2002).
Lieber, C. S., Jones, D. P., Losowsky, M. S. & Davidson, C. S. Interrelation of uric acid and ethanol metabolism in man. J. Clin. Invest. 41, 1863–1870 (1962).
Stiburkova, B., Pavlikova, M., Sokolova, J. & Kozich, V. Metabolic syndrome, alcohol consumption and genetic factors are associated with serum uric acid concentration. PLoS ONE 9, e97646 (2014).
Petrasek J. et al. Metabolic danger signals, uric acid and ATP mediate inflammatory cross-talk between hepatocytes and immune cells in alcoholic liver disease. J. Leukoc. Biol. http://dx.doi.org/10.1189/jlb.3AB1214-590R.
Kono, H. et al. Allopurinol prevents early alcohol-induced liver injury in rats. J. Pharmacol. Exp. Ther. 293, 296–303 (2000).
Ge, X. et al. High mobility group box-1 (HMGB1) participates in the pathogenesis of alcoholic liver disease (ALD). J. Biol. Chem. 289, 22672–22691 (2014).
Yu, M. et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock 26, 174–179 (2006).
Xu, J. et al. Macrophage endocytosis of high-mobility group box 1 triggers pyroptosis. Cell Death Differ. 21, 1229–1239 (2014).
Ganz, M. & Szabo, G. Immune and inflammatory pathways in NASH. Hepatol. Int. 7, 771–781 (2013).
Mehal, W. Z. The inflammasome in liver injury and non-alcoholic fatty liver disease. Dig. Dis. 32, 507–515 (2014).
Lamkanfi, M. & Kanneganti, T. D. The inflammasome: a remote control for metabolic syndrome. Cell Res. 22, 1095–1098 (2012).
Esser, N., Legrand-Poels, S., Piette, J., Scheen, A. J. & Paquot, N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 105, 141–150 (2014).
Lee, H. M. et al. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 62, 194–204 (2013).
Masters, S. L. et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat. Immunol. 11, 897–904 (2010).
Ganz, M., Csak, T. & Szabo, G. High fat diet feeding results in gender specific steatohepatitis and inflammasome activation. World J. Gastroenterol. 20, 8525–8534 (2014).
Wree, A. et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59, 898–910 (2014).
Wree, A. et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J. Mol. Med. (Berl.) 92, 1069–1082 (2014).
Mehal, W. Z. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat. Rev. Gastroenterol. Hepatol. 10, 637–644 (2013).
Pan, J. J. & Fallon, M. B. Gender and racial differences in nonalcoholic fatty liver disease. World J. Hepatol. 6, 274–283 (2014).
Csak, T. et al. Deficiency in myeloid differentiation factor-2 and toll-like receptor 4 expression attenuates nonalcoholic steatohepatitis and fibrosis in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G433–G441 (2011).
Petrasek, J., Csak, T., Ganz, M. & Szabo, G. Differences in innate immune signaling between alcoholic and non-alcoholic steatohepatitis. J. Gastroenterol. Hepatol. 28, 93–98 (2013).
Messina, J. P. et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology 61, 77–87 (2015).
Saha, B. & Szabo, G. Innate immune cell networking in hepatitis C virus infection. J. Leukoc. Biol. 96, 757–766 (2014).
Heim, M. H. Innate immunity and HCV. J. Hepatol. 58, 564–574 (2013).
Dolganiuc, A. et al. Viral and host factors induce macrophage activation and loss of toll-like receptor tolerance in chronic HCV infection. Gastroenterology 133, 1627–1636 (2007).
Chattergoon, M. A. et al. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog. 10, e1004082 (2014).
Chen, W. et al. HCV genomic RNA activates the NLRP3 inflammasome in human myeloid cells. PLoS ONE 9, e84953 (2014).
Burdette, D. et al. Hepatitis C virus activates interleukin-1β via caspase-1-inflammasome complex. J. Gen. Virol. 93, 235–246 (2012).
Jaeschke, H., Williams, C. D., Ramachandran, A. & Bajt, M. L. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 32, 8–20 (2012).
McGill, M. R. et al. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 122, 1574–1583 (2012).
McGill, M. R. et al. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology 60, 1336–1345 (2014).
Williams, C. D., Koerner, M. R., Lampe, J. N., Farhood, A. & Jaeschke, H. Mouse strain-dependent caspase activation during acetaminophen hepatotoxicity does not result in apoptosis or modulation of inflammation. Toxicol. Appl. Pharmacol. 257, 449–458 (2011).
Jaeschke, H., Williams, C. D. & Farhood, A. No evidence for caspase-dependent apoptosis in acetaminophen hepatotoxicity. Hepatology 53, 718–719 (2011).
Williams, C. D., Farhood, A. & Jaeschke, H. Role of caspase-1 and interleukin-1β in acetaminophen-induced hepatic inflammation and liver injury. Toxicol. Appl. Pharmacol. 247, 169–178 (2010).
Sander, L. E. & Blander, J. M. Inflammasome and toll-like receptor 9: Partners in crime in toxic liver injury. Hepatology 49, 2119–2121 (2009).
Chen, C. J. et al. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat. Med. 13, 851–856 (2007).
Hoque, R. et al. P2X7 receptor-mediated purinergic signaling promotes liver injury in acetaminophen hepatotoxicity in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G1171–G1179 (2012).
Williams, C. D. et al. Role of the Nalp3 inflammasome in acetaminophen-induced sterile inflammation and liver injury. Toxicol. Appl. Pharmacol. 252, 289–297 (2011).
Kataoka, H., Kono, H., Patel, Z. & Rock, K. L. Evaluation of the contribution of multiple DAMPs and DAMP receptors in cell death-induced sterile inflammatory responses. PLoS ONE 9, e104741 (2014).
Kono, H., Chen, C. J., Ontiveros, F. & Rock, K. L. Uric acid promotes an acute inflammatory response to sterile cell death in mice. J. Clin. Invest. 120, 1939–1949 (2010).
Antoniades, C. G. et al. Secretory leukocyte protease inhibitor: a pivotal mediator of anti-inflammatory responses in acetaminophen-induced acute liver failure. Hepatology 59, 1564–1576 (2014).
Abu-Amara, M. et al. Liver ischemia/reperfusion injury: processes in inflammatory networks—a review. Liver Transpl. 16, 1016–1032 (2010).
Weigand, K. et al. Ischemia/Reperfusion injury in liver surgery and transplantation: pathophysiology. HPB Surg. 2012, 176723 (2012).
Zhai, Y., Petrowsky, H., Hong, J. C., Busuttil, R. W. & Kupiec-Weglinski, J. W. Ischaemia–reperfusion injury in liver transplantation—from bench to bedside. Nat. Rev. Gastroenterol. Hepatol. 10, 79–89 (2013).
Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 26, 173–179 (2011).
Gabrielli, M. et al. Steatotic livers. Can. we use them in OLTX? Outcome data from a prospective baseline liver biopsy study. Ann. Hepatol. 11, 891–898 (2012).
Farrell, G. C., Teoh, N. C. & McCuskey, R. S. Hepatic microcirculation in fatty liver disease. Anat. Rec. (Hoboken) 291, 684–692 (2008).
DuBray, B. J. Jr. et al. BH3-only proteins contribute to steatotic liver ischemia-reperfusion injury. J. Surg. Res. 194, 653–658 (2014).
Zhu, P. et al. Gene silencing of NALP3 protects against liver ischemia-reperfusion injury in mice. Hum. Gene Ther. 22, 853–864 (2011).
Huang, H. et al. Histones activate the NLRP3 inflammasome in Kupffer cells during sterile inflammatory liver injury. J. Immunol. 191, 2665–2679 (2013).
Kamo, N. et al. ASC/caspase-1/IL-1β signaling triggers inflammatory responses by promoting HMGB1 induction in liver ischemia/reperfusion injury. Hepatology 58, 351–362 (2013).
Shimizu, S. et al. Involvement of ICE family proteases in apoptosis induced by reoxygenation of hypoxic hepatocytes. Am. J. Physiol. 271, G949–G958 (1996).
Shito, M. et al. Interleukin 1 receptor blockade reduces tumor necrosis factor production, tissue injury, and mortality after hepatic ischemia-reperfusion in the rat. Transplantation 63, 143–148 (1997).
Harada, H. et al. Transfer of the interleukin-1 receptor antagonist gene into rat liver abrogates hepatic ischemia-reperfusion injury. Transplantation 74, 1434–1441 (2002).
Takeuchi, D. et al. Interleukin 18 causes hepatic ischemia/reperfusion injury by suppressing anti-inflammatory cytokine expression in mice. Hepatology 39, 699–710 (2004).
Inoue, Y. et al. NLRP3 regulates neutrophil functions and contributes to hepatic ischemia-reperfusion injury independently of inflammasomes. J. Immunol. 192, 4342–4351 (2014).
Watanabe, A. et al. Inflammasome-mediated regulation of hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1248–G1257 (2009).
Ouyang, X., Ghani, A. & Mehal, W. Z. Inflammasome biology in fibrogenesis. Biochim. Biophys. Acta 1832, 979–988 (2013).
Weiskirchen, R. & Tacke, F. Cellular and molecular functions of hepatic stellate cells in inflammatory responses and liver immunology. Hepatobiliary Surg. Nutr. 3, 344–363 (2014).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 (2007).
Khan, F., Peltekian, K. M. & Peterson, T. C. Effect of interferon-α, ribavirin, pentoxifylline, and interleukin-18 antibody on hepatitis C sera-stimulated hepatic stellate cell proliferation. J. Interferon Cytokine Res. 28, 643–651 (2008).
Gieling, R. G., Wallace, K. & Han, Y. P. Interleukin-1 participates in the progression from liver injury to fibrosis. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1324–G1331 (2009).
de Roos, B. et al. Attenuation of inflammation and cellular stress-related pathways maintains insulin sensitivity in obese type I interleukin-1 receptor knockout mice on a high-fat diet. Proteomics 9, 3244–3256 (2009).
Kamari, Y. et al. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J. Hepatol. 55, 1086–1094 (2011).
Isoda, K. et al. Deficiency of interleukin-1 receptor antagonist deteriorates fatty liver and cholesterol metabolism in hypercholesterolemic mice. J. Biol. Chem. 280, 7002–7009 (2005).
Dixon, L. J., Flask, C. A., Papouchado, B. G., Feldstein, A. E. & Nagy, L. E. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS ONE 8, e56100 (2013).
Samstad, E. O. et al. Cholesterol crystals induce complement-dependent inflammasome activation and cytokine release. J. Immunol. 192, 2837–2845 (2014).
Wen, H., Ting, J. P. & O'Neill, L. A. A role for the NLRP3 inflammasome in metabolic diseases—did Warburg miss inflammation? Nat. Immunol. 13, 352–357 (2012).
Ganz, M., Csak, T., Nath, B. & Szabo, G. Lipopolysaccharide induces and activates the Nalp3 inflammasome in the liver. World J. Gastroenterol. 17, 4772–4778 (2011).
Tschopp, J. & Schroder, K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 10, 210–215 (2010).
Petrasek, J. et al. STING-IRF3 pathway links endoplasmic reticulum stress with hepatocyte apoptosis in early alcoholic liver disease. Proc. Natl Acad. Sci. USA 110, 16544–16549 (2013).
Purohit, V. et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol 42, 349–361 (2008).
Neuman, M. G. et al. Mechanisms of alcoholic liver disease: cytokines. Alcohol Clin. Exp. Res. 25, 251S–253S (2001).
Cohen, J. I., Roychowdhury, S., McMullen, M. R., Stavitsky, A. B. & Nagy, L. E. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology 139, 664–674 (2010).
Roychowdhury, S. et al. An early complement-dependent and TLR-4-independent phase in the pathogenesis of ethanol-induced liver injury in mice. Hepatology 49, 1326–1334 (2009).
Sherlock, S. Liver disease in women. Alcohol, autoimmunity, and gallstones. West. J. Med. 149, 683–686 (1988).
Stranges, S. et al. Differential effects of alcohol drinking pattern on liver enzymes in men and women. Alcohol Clin. Exp. Res. 28, 949–956 (2004).
Hatton, J. et al. Drinking patterns, dependency and life-time drinking history in alcohol-related liver disease. Addiction 104, 587–592 (2009).
Askgaard, G. et al. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: a prospective cohort study. J. Hepatol. 62, 1061–1067 (2015).
Csak, T. et al. Mitochondrial antiviral signaling protein defect links impaired antiviral response and liver injury in steatohepatitis in mice. Hepatology 53, 1917–1931 (2011).
Kohli, R. et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 52, 934–944 (2010).
Day, C. P. & James, O. F. Steatohepatitis: a tale of two “hits”? Gastroenterology 114, 842–845 (1998).
Tilg, H. & Moschen, A. R. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52, 1836–1846 (2010).
Linton, S. D. et al. First-in-class pan caspase inhibitor developed for the treatment of liver disease. J. Med. Chem. 48, 6779–6782 (2005).
Shiffman, M. L. et al. Clinical trial: the efficacy and safety of oral PF-03491390, a pancaspase inhibitor—a randomized placebo-controlled study in patients with chronic hepatitis, C. Aliment. Pharmacol. Ther. 31, 969–978 (2010).
Baskin-Bey, E. S. et al. Clinical trial of the pan-caspase inhibitor, IDN-6556, in human liver preservation injury. Am. J. Transplant 7, 218–225 (2007).
Pockros, P. J. et al. Oral IDN-6556, an antiapoptotic caspase inhibitor, may lower aminotransferase activity in patients with chronic hepatitis, C. Hepatology 46, 324–329 (2007).
MacKenzie, S. H., Schipper, J. L. & Clark, A. C. The potential for caspases in drug discovery. Curr. Opin. Drug Discov. Devel. 13, 568–576 (2010).
Church, L. D. & McDermott, M. F. Canakinumab, a fully-human mAb against IL-1β for the potential treatment of inflammatory disorders. Curr. Opin. Mol. Ther. 11, 81–89 (2009).
US National Library of Medicine. ClinicalTrials.gov [online], (2014).
Mandrup-Poulsen, T., Pickersgill, L. & Donath, M. Y. Blockade of interleukin 1 in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 6, 158–166 (2010).
Howard, C. et al. Safety and tolerability of canakinumab, an IL-1β inhibitor, in type 2 diabetes mellitus patients: a pooled analysis of three randomised double-blind studies. Cardiovasc. Diabetol. 13, 94 (2014).
Callus, B. A. & Vaux, D. L. Caspase inhibitors: viral, cellular and chemical. Cell Death Differ. 14, 73–78 (2007).
Lamkanfi, M. et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 187, 61–70 (2009).
Bhaskaracharya, A. et al. Probenecid blocks human P2X7 receptor-induced dye uptake via a pannexin-1 independent mechanism. PLoS ONE 9, e93058 (2014).
Yang, S. J. & Lim, Y. Resveratrol ameliorates hepatic metaflammation and inhibits NLRP3 inflammasome activation. Metabolism 63, 693–701 (2014).
Ambade, A., Catalano, D., Lim, A. & Mandrekar, P. Inhibition of heat shock protein (molecular weight 90 kDa) attenuates proinflammatory cytokines and prevents lipopolysaccharide-induced liver injury in mice. Hepatology 55, 1585–1595 (2012).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Staun-Olsen, P., Bjorneboe, M., Prytz, H., Thomsen, A. C. & Orskov, F. Escherichia coli antibodies in alcoholic liver disease. Correlation to alcohol consumption, alcoholic hepatitis, and serum IgA. Scand. J. Gastroenterol. 18, 889–896 (1983).
Petrasek, J., Csak, T. & Szabo, G. Toll-like receptors in liver disease. Adv. Clin. Chem. 59, 155–201 (2013).
Bala, S., Marcos, M., Gattu, A., Catalano, D. & Szabo, G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS ONE 9, e96864 (2014).
Bode, C. et al. Antibiotics regulate the immune response in both presence and absence of lipopolysaccharide through modulation of Toll-like receptors, cytokine production and phagocytosis in vitro. Int. Immunopharmacol. 18, 27–34 (2014).
Li, P. et al. Mice deficient in IL-1 β-converting enzyme are defective in production of mature IL-1 β and resistant to endotoxic shock. Cell 80, 401–411 (1995).
Wang, S. et al. Murine caspase-11, an ICE-interacting protease, is essential for the activation of ICE. Cell 92, 501–509 (1998).
McGill, M. R. et al. Argininosuccinate synthetase as a plasma biomarker of liver injury after acetaminophen overdose in rodents and humans. Biomarkers 19, 222–230 (2014).
Williams, C. D. et al. Neutrophil activation during acetaminophen hepatotoxicity and repair in mice and humans. Toxicol. Appl. Pharmacol. 275, 122–133 (2014).
McGill, M. R. et al. Circulating acylcarnitines as biomarkers of mitochondrial dysfunction after acetaminophen overdose in mice and humans. Arch. Toxicol. 88, 391–401 (2014).
Author information
Authors and Affiliations
Contributions
G.S. made substantial contributions to discussion of content and reviewed/edited the manuscript before submission. Both authors contributed equally to researching and writing the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Szabo, G., Petrasek, J. Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 12, 387–400 (2015). https://doi.org/10.1038/nrgastro.2015.94
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrgastro.2015.94
This article is cited by
-
Verapamil-loaded supramolecular hydrogel patch attenuates metabolic dysfunction-associated fatty liver disease via restoration of autophagic clearance of aggregated proteins and inhibition of NLRP3
Biomaterials Research (2023)
-
IL-33/ST2 antagonizes STING signal transduction via autophagy in response to acetaminophen-mediated toxicological immunity
Cell Communication and Signaling (2023)
-
Tanshinone I specifically suppresses NLRP3 inflammasome activation by disrupting the association of NLRP3 and ASC
Molecular Medicine (2023)
-
Exploring immune related gene signatures and mechanisms linking non alcoholic fatty liver disease to atrial fibrillation through transcriptome data analysis
Scientific Reports (2023)
-
Gut microbiota regulates chronic ethanol exposure-induced depressive-like behavior through hippocampal NLRP3-mediated neuroinflammation
Molecular Psychiatry (2023)