Abstract
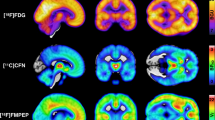
Functional neuroimaging techniques can be used to study changes in regional brain activation, using changes in surrogate markers such as regional cerebral perfusion and rates of glucose uptake or metabolism. These approaches are shedding new light on two major health problems: the increasing burden of type 2 diabetes mellitus (T2DM), which is driven by the rising prevalence of insulin resistance and obesity; and recurrent intractable problematic hypoglycaemia, which is driven by the cognitive impairment that can occur in association with iatrogenic hypoglycaemic episodes. Some patients with diabetes mellitus lose awareness of being hypoglycaemic, which puts them at risk of severe hypoglycaemia as they are unlikely to take action to prevent the condition worsening. Involvement of corticolimbic brain and centres serving higher executive functions as well as the hypothalamus has been demonstrated in both situations and has implications for therapy. This Review describes the relevant principles of functional neuroimaging techniques and presents data supporting the notion that the dysregulation of central pathways involved in metabolic regulation, reward and appetite could contribute to problematic hypoglycaemia during therapy for diabetes mellitus and to insulin-resistant obesity and T2DM. Understanding these dysregulations could enable the development of novel clinical interventions.
Key Points
-
Functional neuroimaging shows brain regions that are activated during task performance or in response to stimuli by detecting changes in markers showing, for example, perfusion, nutrient uptake or metabolism
-
Functional neuroimaging has been used to study therapy-related hypoglycaemia in patients with diabetes mellitus and the increasing prevalence of type 2 diabetes mellitus (T2DM), which is driven by obesity
-
Patients with acute hypoglycaemia demonstrate extensive activation of the cortex, putative glucose-sensing brain regions and stress pathways; these features are altered in patients with counter-regulatory deficits and hypoglycaemia unawareness
-
In patients with hypoglycaemia unawareness, altered responses of reward and memory pathways might prevent long-term restoration of awareness
-
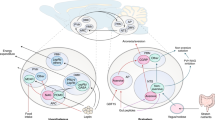
Growing evidence from functional neuroimaging studies indicates that dysfunction of reward and motivational pathways occurs in individuals with obesity or T2DM
-
Increased understanding of the cortical components of these alterations in patients with T2DM, obtained from neuroimaging studies, could lead to development of effective therapeutic interventions
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bernard, C. I. J. An Introduction to the Study of Experimental Medicine (Macmillan & Co. Ltd, New York, 1927).
Roy, C. S. & Sherrington, C. S. On the regulation of the blood supply of the brian. J. Physiol. 11, 85–108 (1890).
Metea, M. R. & Newman, E. A. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J. Neurosci. 26, 2862–2870 (2006).
Figley, C. R. & Stroman, P. W. The role(s) of astrocytes and astrocyte activity in neurometabolism, neurovascular coupling, and the production of functional neuroimaging signals. Eur. J. Neurosci. 33, 577–588 (2011).
Phelps, M. E. et al. Tomographic measurement of local cerebral glucose metabolic rate in humans with 182-fluoro-2-deoxy-D-glucose: validation of method. Ann. Neurol. 6, 371–388 (1979).
Kuwabara, H., Brust, P., Steinbach, J. & Bergmann, R. Blood–brain transport and metabolic rate of glucose measured with 11C-O-methyl-D-glucose (OMG) [abstract 73P]. J. Nucl. Med. 40 (suppl.), 293 (1999).
Nakanishi, H., Cruz, N. F., Adachi, K., Sokoloff, L. & Dienel, G. A. Influence of glucose supply and demand on determination of brain glucose content with labelled methylglucose. J. Cereb. Blood Flow Metab. 16, 439–449 (1996).
Raichle, M. E., Martin, W. R., Herscovitch, P., Mintun, M. A. & Markham, J. Brain blood flow measured with intravenous H215O. II. Implementation and validation. J. Nucl. Med. 24, 790–798 (1983).
Finnema, S. J., Bang-Andersen, B., Wikström, H. V. & Halldin, C. Current state of agonist radioligands for imaging of brain dopamine D2/D3 receptors in vivo with positron emission tomography. Curr. Top. Med. Chem. 10, 1477–1498 (2010).
Ogawa, S., Lee, T. M., Kay, A. R. & Tank, D. W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl Acad. Sci. USA 87, 9868–9872 (1990).
Malonek, D. & Grinvald, A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science 272, 551–554 (1996).
Goldstone, A. P. et al. Fasting biases brain reward systems towards high-calorie foods. Eur. J. Neurosci. 30, 1625–1635 (2009).
Williams, D. S. Quantitative perfusion imaging using arterial spin labelling. Methods Mol. Med. 124, 151–173 (2006).
Wong, E. C. Quantifying CBF with pulsed ASL: technical and pulse sequence factors. J. Magn. Reson. Imaging 22, 727–731 (2005).
Dai, W., Garcia, D., de Bazelaire, C. & Alsop, D. C. Continuous flow-driven inversion for arterial spin labelling using pulsed radio frequency and gradient fields. Magn. Reson. Med. 60, 1488–1497 (2008).
Mitrakou, A. et al. Hierarchy of glycaemic thresholds for counter-regulatory hormone secretion, symptoms, and cerebral dysfunction. Am. J. Physiol. 260, E67–E74 (1991).
Gerich, J., Cryer, P. & Rizza, R. Hormonal mechanisms in acute glucose counter-regulation: the relative roles of glucagon, epinephrine, norepinephrine, growth hormone, and cortisol. Metab. Clin. Exp. 29, 1164–1175 (1980).
Kerr, D. et al. Symmetry of cerebral blood flow and cognitive responses to hypoglycaemia in humans. Diabetologia 36, 73–78 (1993).
Bolli, G. B. et al. Abnormal glucose counter-regulation after subcutaneous insulin in insulin-dependent diabetes mellitus. N. Engl. J. Med. 310, 1706–1711 (1984).
Peacey, S. R., Rostami-Hodjegan, A., George, E., Tucker, G. T. & Heller, S. R. The use of tolbutamide-induced hypoglycemia to examine the intraislet role of insulin in mediating glucagon release in normal humans. J. Clin. Endocrinol. Metab. 82, 1458–1461 (1997).
Deary, I. J., Hepburn, D. A., MacLeod, K. M. & Frier, B. M. Partitioning the symptoms of hypoglycaemia using multi-sample confirmatory factor analysis. Diabetologia. 36, 771–777 (1993).
Lawrence, R. Insulin hypoglycaemia changes in nervous manifestations. Lancet 238, 602 (1941).
Geddes, J., Schopman, J. E., Zammitt, N. N. & Frier, B. M. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet. Med. 25, 501–504 (2008).
Schopman, J. E., Geddes, J. & Frier, B. M. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res. Clin. Pract. 87, 64–68 (2010).
Maran, A., Taylor, J., Macdonald, I. A. & Amiel, S. A. Evidence for reversibility of defective counter-regulation in a patient with insulinoma. Diabet. Med. 9, 765–768 (1992).
Cranston, I., Lomas, J., Maran, A., Macdonald, I. & Amiel, S. A. Restoration of hypoglycaemia awareness in patients with long-duration insulin-dependent diabetes. Lancet 344, 283–287 (1994).
Fanelli, C. et al. Long-term intensive therapy of IDDM patients with clinically overt autonomic neuropathy: effects on hypoglycemia awareness and counter-regulation. Diabetes 46, 1172–1181 (1997).
Dagogo-Jack, S., Rattarasarn, C. & Cryer, P. E. Reversal of hypoglycemia unawareness, but not defective glucose counter-regulation, in IDDM. Diabetes 43, 1426–1434 (1994).
Mühlhauser, I. et al. Bicentric evaluation of a teaching and treatment programme for type 1 (insulin-dependent) diabetic patients: improvement of metabolic control and other measures of diabetes care for up to 22 months. Diabetologia 25, 470–476 (1983).
Sämann, A., Mühlhauser, I., Bender, R., Kloos, C. & Müller, U. A. Glycaemic control and severe hypoglycaemia following training in flexible, intensive insulin therapy to enable dietary freedom in people with type 1 diabetes: a prospective implementation study. Diabetologia 48, 1965–1970 (2005).
Hopkins, D. et al. Improved biomedical and psychological outcomes 1 year after structured education in flexible insulin therapy for people with type 1 diabetes mellitus: the UK DAFNE Experience. Diabetes Care http://dx.doi.org/10.2337/dc11-1579.
Page, K. A. et al. Small decrements in systemic glucose provoke increases in hypothalamic blood flow prior to the release of counter-regulatory hormones. Diabetes 58, 448–452 (2009).
Musen, G. et al. Regional brain activation during hypoglycemia in type 1 diabetes. J. Clin. Endocrinol. Metab. 93, 1450–1457 (2008).
Teh, M. M. et al. Evolution and resolution of human brain perfusion responses to the stress of induced hypoglycemia. Neuroimage 53, 584–592 (2010).
Jauch-Chara, K. et al. Hypoglycemia during sleep impairs consolidation of declarative memory in type 1 diabetes and healthy humans, Diabetes Care 30, 2040–2045 (2007).
Warren, R. E., Zammitt, N. N, Deary, I. J & Frier, B. M. The effects of acute hypoglycaemia on memory acquisition and recall and prospective memory in type 1 diabetes. Diabetologia 50, 178–185 (2007).
Uehara, Y., Nipper, V. & McCall, A. L. Chronic insulin hypoglycemia induces GLUT-3 protein in rat brain neurons. Am. J. Physiol. 272, E716–E719 (1997).
Kumagai, A. K., Kang, Y. S., Boado, R. J. & Pardridge, W. M. Upregulation of blood–brain barrier GLUT1 glucose transporter protein and mRNA in experimental chronic hypoglycemia. Diabetes 44, 1399–1404 (1995).
Kety, S. & Schmidt, C. The nitrous oxide method for the quantitative determination of cerebral blood flow in man; theory, procedure and normal values. J. Clin. Invest. 27, 476–483 (1948).
Boyle, P. J. et al. Adaptation in brain glucose uptake following recurrent hypoglycemia. Proc. Natl Acad. Sci. USA 91, 9352–9356 (1994).
Boyle, P. J., Kempers, S. F., O'Connor, A. M. & Nagy, R. J. Brain glucose uptake and unawareness of hypoglycemia in patients with insulin-dependent diabetes mellitus. N. Engl. J. Med. 333, 1726–1731 (1995).
Amiel, S. A. et al. Effect of antecedent glucose control on cerebral function during hypoglycemia. Diabetes Care 14, 109–118 (1991).
Maran, A., Lomas, J., Macdonald, I. A. & Amiel, S. A. Lack of preservation of higher brain function during hypoglycaemia in patients with intensively-treated IDDM. Diabetologia 38, 1412–1418 (1995).
Hvidberg, A. et al. Impact of recent antecedent hypoglycemia on hypoglycaemic cognitive dysfunction in nondiabetic humans. Diabetes 45, 1030–1036 (1996).
Ovalle, F. et al. Brief twice-weekly episodes of hypoglycemia reduce detection of clinical hypoglycemia in type 1 diabetes mellitus. Diabetes 47, 1472–1479 (1998).
Fanelli, C. G. et al. Impact of nocturnal hypoglycemia on hypoglycaemic cognitive dysfunction in type 1 diabetes. Diabetes 47, 1920–1927 (1998).
Spyer, G., Hattersley, A. T., MacDonald, I. A., Amiel, S. & MacLeod, K. M. Hypoglycaemic counter-regulation at normal blood glucose concentrations in patients with well controlled type 2 diabetes. Lancet 356, 1970–1974 (2000).
Brooks, D. J. et al. Regional cerebral glucose transport in insulin-dependent diabetic patients studied using 11C 3-O-methyl-D-glucose and positron emission tomography. J. Cereb. Blood Flow Metab. 6, 240–244 (1986).
Fanelli, C. G. et al. Blood-to-brain glucose transport and cerebral glucose metabolism are not reduced in poorly controlled type 1 diabetes. Diabetes 47, 1444–1450 (1998).
Segel, S. A. et al. Blood-to-brain glucose transport, cerebral glucose metabolism, and cerebral blood flow are not increased after hypoglycemia. Diabetes 50, 1911–1917 (2001).
Bingham, E. M. et al. Differential changes in brain glucose metabolism during hypoglycaemia accompany loss of hypoglycaemia awareness in men with type 1 diabetes mellitus. An 11C-3-O-methyl-D-glucose PET study. Diabetologia 48, 2080–2089 (2005).
Cranston, I., Reed, L. J., Marsden, P. K. & Amiel, S. A. Changes in regional brain 18F-fluorodeoxyglucose uptake at hypoglycemia in type 1 diabetic men associated with hypoglycemia unawareness and counter-regulatory failure. Diabetes 50, 2329–2336 (2001).
Dunn, J. T., Cranston, I., Marsden, P. K., Amiel, S. A. & Reed, L. J. Attenuation of amygdala and frontal cortical responses to low blood glucose concentration in asymptomatic hypoglycemia in type 1 diabetes: a new player in hypoglycemia unawareness? Diabetes 56, 2766–2773 (2007).
Arbelaez, A. M., Powers, W. J., Videen, T. O., Price, J. L. & Cryer, P. E. Attenuation of counter-regulatory responses to recurrent hypoglycemia by active thalamic inhibition: a mechanism for hypoglycemia-associated autonomic failure. Diabetes 57, 470–475 (2008).
Smith, C. B., Choudhary, P., Pernet, A., Hopkins, D. & Amiel, S. A. Hypoglycemia unawareness is associated with reduced adherence to therapeutic decisions in patients with type 1 diabetes: evidence from a clinical audit. Diabetes Care 32, 1196–1198 (2009).
Rogers, H., de Zoysa, N. & Amiel, S. Patient experience of hypoglycaemia unawareness in type 1 diabetes: are patients appropriately concerned? Diabet. Med. 29, 321–327 (2012).
Rosenthal, J. M. et al. The effect of acute hypoglycemia on brain function and activation: a functional magnetic resonance imaging study. Diabetes 50, 1618–1626 (2001).
Bolo, N. R. et al. Brain activation during working memory is altered in patients with type 1 diabetes during hypoglycemia. Diabetes 60, 3256–3264 (2011).
Rosenthal, M. J. et al. Caffeine restores regional brain activation in acute hypoglycaemia in healthy volunteers. Diabet. Med. 24, 720–727 (2007).
International Diabetes Federation. Diabetes Atlas, 5th edition. [online], (2011).
Thorleifsson, G. et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat. Genet. 41, 18–24 (2009).
Willer, C. et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat. Genet. 41, 25–34 (2009).
Matsuda, M. et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 48, 1801–1806 (1999).
Liu, Y., Gao, J.-H., Liu, H.-L. & Fox, P. T. The temporal response of the brain after eating revealed by functional MRI. Nature 405, 1058–1062 (2000).
Smeets, P. A. M., de Graaf, C., Stafleu, A., van Osch, M. J. P. & van der Grond, J. Functional MRI of human hypothalamic responses following glucose ingestion. Neuroimage 24, 363–368 (2005).
Vidarsdottir, S. et al. Glucose ingestion fails to inhibit hypothalamic neuronal activity in patients with type 2 diabetes. Diabetes 56, 2547–2550 (2007).
Berridge, K. C., Ho, C. Y., Richard, J. M. & DiFeliceantonio, A. G. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res. 1350, 43–64 (2010).
Cranston, I. et al. Regional differences in cerebral blood flow and glucose utilization in diabetic man: the effect of insulin. J. Cereb. Blood Flow Metab. 18, 130–140 (1998).
Hasselbalch, S. G. et al. No effect of insulin on glucose blood–brain barrier transport and cerebral metabolism in humans. Diabetes 48, 1915–1921 (1999).
Bondy, C. A & Cheng, C. M. Signalling by insulin-like growth factor 1 in brain. Eur. J. Pharmacol. 490, 25–31 (2004).
Bingham, E. M. et al. The role of insulin in human brain glucose metabolism. Diabetes 51, 3384–3390 (2002).
Anthony, K. et al. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance. Diabetes 55, 2986–2992 (2006).
Berthoud, H.-R. & Morrison, C. The brain, appetite, and obesity. Annu. Rev. Psychol. 59, 55–92 (2008).
Zhu, J. N. & Wang, J. J. The cerebellum in feeding control: possible function and mechanism. Cell. Mol. Neurobiol. 28, 469–478 (2008).
Figlewicz, D. P. & Benoit, S. C. Insulin, leptin, and food reward: update 2008. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R9–R19 (2008).
Wang, G.-J. et al. Enhanced resting activity of the oral somatosensory cortex in obese subjects. Neuroreport 13, 1151–1155 (2002).
Kullmann, S. et al. The obese brain: association of body mass index and insulin sensitivity with resting state network functional connectivity. Hum. Brain Mapp. 33, 1052–1061 (2012).
Hallschmid, M., Benedict, C., Schultes, B., Born, J. & Kern, W. Obese men respond to cognitive but not to catabolic brain insulin signalling. Int. J. Obes. (Lond.) 32, 275–282 (2008).
Hallschmid, M. et al. Intranasal insulin reduces body fat in men but not in women. Diabetes 53, 3024–3029 (2004).
Benedict, C., Kern, W., Schultes, B., Born, J. & Hallschmid, M. Differential sensitivity of men and women to anorexigenic and memory-improving effects of intranasal insulin. J. Clin. Endocrinol. Metab. 93, 1339–1344 (2008).
Benedict, C. et al. Intranasal insulin enhances postprandial thermogenesis and lowers postprandial serum insulin levels in healthy men. Diabetes 60, 114–118 (2011).
Hallschmid, M., Higgs, S., Thienel, M., Ott, V. & Lehnert, H. Postprandial administration of intranasal insulin intensifies satiety and reduces intake of palatable snacks in women. Diabetes 61, 782–789 (2012).
Tschritter, O. et al. The cerebrocortical response to hyperinsulinaemia is reduced in overweight humans: a magnetoencephalographic study. Proc. Natl Acad. Sci. 103, 12103–12108 (2006).
Tschritter, O. et al. Variation in the FTO gene locus is associated with cerebrocortical insulin resistance in humans. Diabetologia 50, 2602–2603 (2007).
Tschritter, O. et al. High cerebral insulin sensitivity is associated with loss of body fat during lifestyle intervention. Diabeteologia 55, 175–182 (2012).
Amiel, S. A., Sherwin, R. S., Simonson, D. C., Lauritano, A. A. & Tamborlane, W. V. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N. Engl. J. Med. 315, 215–219 (1986).
Amiel, S. A. et al. Insulin resistance of puberty: a defect restricted to peripheral glucose metabolism. J. Clin. Endocrinol. Metab. 72, 277–282 (1991).
Wallner-Liebmann, S. et al. Insulin and hippocampus activation in response to images of high-calorie food in normal weight and obese adolescents. Obesity (Silver Spring) 18, 1552–1557 (2010).
Le, D. S. N. et al. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am. J. Clin. Nutr. 84, 725–731 (2006).
Le, D. S. N. et al. Less activation in the left dorsolateral prefrontal cortex in the reanalysis of the response to a meal in obese than in lean women and its association with successful weight loss. Am. J. Clin. Nutr. 86, 573–579 (2007).
DelParigi, A. et al. Persistence of abnormal neural responses to a meal in postobese individuals. Int. J. Obes. Relat. Metab. Disord. 28, 370–377 (2004).
van de Sande-Lee, S. et al. Partial reversibility of hypothalamic dysfunction and changes in brain activity after body mass reduction in obese subjects. Diabetes 60, 1699–1704 (2011).
van der Laan, L. N., de Ridder, D. T., Viergever, M. A & Smeets, P. A. The first taste is always with the eyes: a meta-analysis on the neural correlates of processing visual food cues. Neuroimage 57, 296–303 (2011).
Small, D. M. & Prescott, J. Odour/taste integration and the perception of flavour. Exp. Brain Res. 166, 345–357 (2005).
Rothemund, Y. et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage 37, 410–421 (2007).
Stoeckel, L. E. et al. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage 41, 636–647 (2008).
Martin, L. E. et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity (Silver Spring) 18, 254–260 (2010).
Guthoff, M. et al. The insulin-mediated modulation of visually evoked magnetic fields is reduced in obese subjects. PLoS ONE 6, e19482 (2011).
Stoeckel, L. E. et al. Effective connectivity of a reward network in obese women. Brain Res. Bull. 79, 388–395 (2009).
Cornier, M.-A. et al. The effects of overfeeding on the neuronal response to visual food cues in thin and reduced-obese individuals. PLoS ONE 4, e6310 (2009).
Zhou, H. et al. Impairments in cognition and resting-state connectivity of the hippocampus in elderly subjects with type 2 diabetes. Neurosci. Lett. 473, 5–10 (2010).
Chechlacz, M. et al. Diabetes dietary management alters responses to food pictures in brain regions associated with motivation and emotion: a functional magnetic resonance imaging study. Diabetologia 52, 524–533 (2009).
Volkow, N. D. et al. 'Nonhedonic' food motivation in humans involves dopamine in the dorsal striatum and methylphenidate amplifies this effect. Synapse 44, 175–180 (2002).
Chen, P. S. et al. Correlation between body mass index and striatal dopamine transporter availability in healthy volunteers—a SPECT study. Neuroimage 40, 275–279 (2008).
Wang, G. J. et al. Brain dopamine and obesity. Lancet 357, 354–357 (2001).
Volkow, N. D. et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42, 1537–1543 (2008).
Haltia, L. T. et al. Effects of intravenous glucose on dopaminergic function in the human brain in vivo. Synapse 61, 748–756 (2007).
Killgore, W. D. S. et al. Citicoline affects appetite and corticolimbic responses to images of high-calorie foods. Int. J. Eat. Disord. 43, 6–13 (2010).
Baker, L. D. et al. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch. Neurol. 68, 51–57 (2011).
Seaquist, E. R. et al. Insulin reduces the BOLD response but is without effect on the VEP during presentation of a visual task in humans. J. Cereb. Blood Flow Metab. 27, 154–160 (2007).
Guthoff, M. et al. Insulin modulates food-related activity in the central nervous system. JCEM 95, 748–755 (2010).
Acknowledgements
The authors acknowledge grant support from the Wellcome Trust; Juvenile Diabetes Research Foundation; the Comprehensive Biomedical Research Centre of Guy's and St Thomas' NHS Foundation Trust, and King's College London and the King's College Hospital Charity.
Author information
Authors and Affiliations
Contributions
Y.-S. Cheah and S. A. Amiel contributed equally to researching the data for the article, discussions of its content, writing the article, and reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cheah, YS., Amiel, S. Metabolic neuroimaging of the brain in diabetes mellitus and hypoglycaemia. Nat Rev Endocrinol 8, 588–597 (2012). https://doi.org/10.1038/nrendo.2012.97
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2012.97
This article is cited by
-
Brain glucose metabolism during hypoglycemia in type 1 diabetes: insights from functional and metabolic neuroimaging studies
Cellular and Molecular Life Sciences (2016)