Abstract
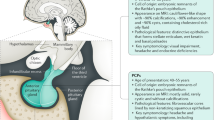
Craniopharyngiomas have an overall incidence of 0.5–2.0 new cases per million of the population per year, and ∼30–50% of all cases represent childhood craniopharyngioma. These partly cystic embryogenic malformations of the sellar region are presumably derived from Rathke cleft epithelium. Many of the typical manifestations at primary diagnosis are nonspecific and include headache, visual impairment, polyuria and/or polydypsia, growth retardation and weight gain. Total resection is the treatment of choice in patients with favorable tumor localization, with the intention to maintain hypothalamic–pituitary and optical nerve functions. When the tumor localization is unfavorable, a limited resection followed by local irradiation is recommended. The overall survival rates are high (91–98%). High recurrence rates after complete resection and high progression rates after incomplete resection have been observed, although the risk of recurrence or progression is less after complete resection than partial resection. Irradiation of the tumor is protective and the appropriate time point of irradiation after incomplete resection is currently under investigation in a randomized trial. Long-term sequelae substantially reduce the quality of life of ∼50% of long-term survivors, notably extreme obesity owing to hypothalamic involvement.
Key Points
-
The combination of headache, visual impairment, pathological low growth rate, weight gain and polydipsia and/or polyuria should arouse suspicion of craniopharyngioma in the differential diagnosis
-
A pathological low growth rate demonstrable as early as 12 months of age can be an early manifestation of the disease; an increase in weight tends to occur as a late manifestation
-
The combination of solid, cystic and calcified craniopharyngioma components is an important radiological clue to the tumor diagnosis on MRI and CT
-
Quality of life is frequently impaired in long-term survivors due to sequelae caused by the anatomical proximity of craniopharyngioma to the optic nerve and/or chiasma, pituitary gland and hypothalamus
-
Extreme obesity owing to hypothalamic involvement has a major negative impact on quality of life
-
For unfavorably localized craniopharyngiomas with hypothalamic involvement, a radical neurosurgical treatment strategy is not recommended owing to the high risk of adverse sequelae
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Müller, H. L. Childhood craniopharyngioma. Recent advances in diagnosis, treatment and follow-up. Horm. Res. 69, 193–202 (2008).
Müller, H. L. More or less? Treatment strategies in childhood craniopharyngioma. Childs Nerv. Syst. 22, 156–157 (2006).
Müller, H. L. et al. Consensus and perspectives on treatment strategies in childhood craniopharyngioma—results of a meeting of the Craniopharyngioma Study Group (SIOP), Genova 2004. J. Pediatric Endocrinol. Metab. 19 (Suppl. 1), 453–454 (2006).
Garrè, M. L. & Cama, A. Craniopharyngioma: modern concepts in pathogenesis and treatment. Curr. Opin. Pediatr. 19, 471–479 (2007).
Bunin, G. R. et al. The descriptive epidemiology of craniopharyngioma. Neurosurg. Focus 15, e1 (1997).
Müller-Scholden, J. et al. Radical surgery in a neonate with craniopharyngioma. Report of case. Pediatr. Neurosurg. 33, 265–269 (2000).
Rushing, E. J., Giangaspero, F., Paulus, W. & Burger, P. C. In WHO Classification of Tumours of the Central Nervous System (eds Louis, D. N., Ohgaki, H., Wiestler, O. D. & Cavanee, W. K) 238–240 (IARC Press, Lyon, 2007).
Sato, K. et al. Ciliated craniopharyngioma may arise from Rathke cleft cyst. Clin. Neuropathol. 25, 25–28 (2006).
Hölsken, A., Buchfelder, M., Fahlbusch, R., Blümcke, I. & Buslei, R. Tumour cell migration in adamantinomatous craniopharyngiomas is promoted by activated Wnt-signalling. Acta Neuropathol. 119, 631–639 (2010).
Buslei, R. et al. Common mutations of beta-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol. 109, 589–597 (2005).
Kato, K. et al. Possible linkage between specific histological structures and aberrant reactivation of the Wnt pathway in adamantinomatous craniopharyngioma. J. Pathol. 203, 814–821 (2004).
Oikonomou, E. et al. Beta-catenin mutations in craniopharyngiomas and pituitary adenomas. J. Neurooncol. 73, 205–209 (2005).
Sekine, S. et al. Craniopharyngiomas of adamantinomatous type harbor beta-catenin gene mutations. Am. J. Pathol. 161, 1997–2001 (2002).
Hassanein, A. M., Glanz, S. M., Kessler, H. P., Eskin, T. A. & Liu, C. beta-Catenin is expressed aberrantly in tumors expressing shadow cells. Pilomatricoma, craniopharyngioma, and calcifying odontogenic cyst. Am. J. Clin. Pathol. 120, 732–736 (2003).
Hofmann, B. M. et al. Nuclear beta-catenin accumulation as reliable marker for the differentiation between cystic craniopharyngiomas and rathke cleft cysts: a clinico-pathologic approach. Am. J. Surg. Pathol. 30, 1595–1603 (2006).
Müller, H. L., Kaatsch, P., Warmuth-Metz, M., Flentje, M. & Sörensen, N. Childhood craniopharyngioma—diagnostic and therapeutic strategies. Monatsschr Kinderheilkunde 151, 1056–1063 (2003).
Müller, H. L. et al. Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 89, 3298–3305 (2004).
Müller, H. L. et al. Functional capacity and body mass index in patients with sellar masses—cross-sectional study on 403 patients diagnosed during childhood and adolescence. Childs Nerv. Syst. 21, 539–545 (2005).
Warmuth-Metz, M., Gnekow, A. K., Müller, H. & Solymosi, L. Differential diagnosis of suprasellar tumors in children. Klin. Padiatr. 216, 323–330 (2004).
Rennert, J. & Doerfler, A. Imaging of sellar and parasellar lesions. Clin. Neurol. Neurosurg. 109, 111–124 (2007).
Rossi, A. et al. Neuroimaging of pediatric craniopharyngiomas: a pictorial essay. J. Pediatr. Endocrinol. Metab. 19 (Suppl. 1), 299–319 (2006).
Choux, M., Lena, G. & Genitori, L. Craniopharyngioma in children. Neurochirurgie 37 (Suppl. 1), 1–174 (1991).
Fahlbusch, R., Honegger, J., Paulus, W., Huk, W. & Buchfelder, M. Surgical management of craniopharyngiomas: Experience with 168 patients. J. Neurosurg. 90, 237–250 (1999).
Zona, G. & Spaziante, R. Management of cystic craniopharyngiomas in childhood by a transsphenoidal approach. J. Pediatr. Endocrinol. Metab. 19 (Suppl. 1), 381–388 (2006).
Becker, G., Kortmann, R. D., Skalej, M. & Bamberg, M. The role of radiotherapy in the treatment of craniopharyngioma—indications, results, side effects/ Front. Radiat. Ther. Oncol. 33, 100–113 (1999).
Einhaus, S. L. & Sanford, R. A. In Principles and Practice of Pediatric Neurosurgery (eds Albright, A. L., Pollack, I. F. & Adelson, P. D.) 545–562 (Thieme Medical Publishers, New York, 2007).
Elliott, R. E. et al. Efficacy and safety of radical resection of primary and recurrent craniopharyngiomas in 86 children. J. Neurosurg. Pediatr. 5, 30–48 (2010).
Puget, S. et al. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J. Neurosurg. 106 (Suppl. 1), 3–12 (2007).
Gupta, D. K., Ojha, B. K., Sarkar, C., Mahapatra, A. K. & Mehta, V. S. Recurrence in craniopharyngiomas: analysis of clinical and histological features. J. Clin. Neurosci. 13, 438–442 (2006).
Karavitaki, N. et al. Craniopharyngiomas in children and adults: systematic analysis of 121 cases with long-term follow-up. Clin. Endocrinol. (Oxf.) 62, 397–409 (2005).
Dhellemmes, P. & Vinchon, M. Radical resection for craniopharyngiomas in children: surgical technique and clinical results. J. Pediatr. Endocrinol. Metab. 19 (Suppl. 1), 329–335 (2006).
Vinchon, M., Weill, J., Delestret, I. & Dhellemmes, P. Craniopharyngioma and hypothalamic obesity in children. Childs Nerv. Syst. 25, 347–352 (2009).
Fischer, E. G., Welch, K., Shillito, J. Jr, Winston, K. R. & Tarbell, N. J. Craniopharyngiomas in children. Long-term effects of conservative surgical procedures combined with radiation therapy. J. Neurosurg. 73, 534–540 (1990).
Hetelekidis, S. et al. 20-year experience in childhood craniopharyngioma. Int. J. Radiat. Oncol. Biol. Phys. 27, 189–195 (1993).
Rajan, B. et al. Craniopharyngioma—long-term results following limited surgery and radiotherapy. Radiother. Oncol. 26, 1–10 (1993).
Merchant, T. E. et al. Craniopharyngioma: the St Jude Children's Research Hospital experience 1984–2001. Int. J. Radiat. Oncol. Biol. Phys. 53, 533–542 (2002).
Tomita, T. & Bowman, R. M. Craniopharyngiomas in children: surgical experience at Children's Memorial Hospital. Childs Nerv. Syst. 21, 729–746 (2005).
Yasargil, M. G. et al. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J. Neurosurg. 73, 3–11 (1990).
Schubert, T. et al. Neurosurgical treatment strategies in childhood craniopharyngiomas: is less more? Childs Nerv. Syst. 25, 1419–1427 (2009).
Müller, H. L. et al. Analyses of treatment variables for patients with childhood craniopharyngioma—results of the multicenter prospective study trial KRANIOPHARYNGEOM 2000 after three years of follow up. Horm. Res. Paediatr. 73, 175–180 (2010).
Sanford, R. A. Craniopharyngioma: results of survey of the American Society of Pediatric Neurosurgery. Pediatr. Neurosurg. 21 (Suppl. 1), 39–43 (1994).
Wolff, J. E. et al. Münster Heidelberg Abilities Scale—a measuring instrument for global comparison of illness sequelae [in German]. Klin. Padiatr. 208, 294–298 (1996).
Fitzek, M. M., Linggood, R. M., Adams, J. & Munzenrider, J. E. Combined proton and photon irradiation for craniopharyngioma: long-term results of the early cohort of patients treated at Harvard Cyclotron Laboratory and Massachusetts General Hospital. Int. J. Radiat. Oncol. Biol. Phys. 64, 1348–1354 (2006).
Luu, Q. T. et al. Fractionated proton radiation treatment for pediatric craniopharyngioma: preliminary report. Cancer J. 12, 155–159 (2006).
Baumert, B. G., Norton, I. A., Lomax, A. J. & Davis, J. B. Dose conformation of intensity-modulated stereotactic photon beams, proton beams, and intensity-modulated proton beams for intracranial lesions. Int. J. Radiat. Oncol. Biol. Phys. 60, 1314–1324 (2004).
Habrand, J. L. et al. Radiation therapy in the management of craniopharyngioma: current concepts and future developments. J. Pediatr. Endocrinol. Metab. 19 (Suppl. 1), 389–394 (2006).
Minniti, G. et al. Fractionated stereotactic conformal radiotherapy following conservative surgery in the control of craniopharyngiomas. Radiother. Oncol. 82, 90–95 (2007).
Jalali, R. et al. Factors influencing neurocognitive outcomes in young patients with benign and low-grade brain tumors treated with stereotactic conformal radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 77, 974–979 (2010).
Merchant, T. E. et al. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J. Neurosurg. 104 (Suppl. 2), 94–102 (2006).
Combs, S. E. et al. Achievement of long-term local control in patients with craniopharyngiomas using high precision stereotactic radiotherapy. Cancer 109, 2308–2314 (2007).
Hasegawa, T., Kobayashi, T. & Kida, Y. Tolerance of the optic apparatus in single-fraction irradiation using stereotactic radiosurgery: evaluation in 100 patients with craniopharyngioma. Neurosurgery 66, 688–694 (2010).
Kobayashi, T. Long-term results of gamma knife radiosurgery for 100 consecutive cases of craniopharyngioma and a treatment strategy. Prog. Neurol. Surg. 22, 63–76 (2009).
Julow, J. et al. Long-term results and late complications after intracavitary yttrium-90 colloid irradiation of recurrent cystic craniopharyngiomas. Neurosurgery 61, 288–295 (2007).
Szeifert, G. T. et al. Pathological findings in cystic craniopharyngiomas after stereotactic intracavitary irradiation with yttrium-90 isotope. Prog. Neurol. Surg. 20, 297–302 (2007).
Cavalhero, S., Sparapani, F. V., Franco, J. O., da Silva, M. C. & Braga, F. M. Use of bleomycin in intratumoral chemotherapy for cystic craniopharyngioma. J. Neurosurg. 84, 124–126 (1996).
Kim, S. D. et al. Radiological findings following postsurgical intratumoral bleomycin injection for cystic craniopharyngioma. Clin. Neurol. Neurosurg. 109, 236–241 (2007).
Ierardi, D. F. et al. Apoptosis in alpha interferon (IFN-alpha) intratumoral chemotherapy for cystic craniopharyngiomas. Childs Nerv. Syst. 23, 1041–1046 (2007).
Defoort-Dhellemmes, S., Moritz, F., Bouacha, I. & Vinchon, M. Craniopharyngioma: ophthalmological aspects at diagnosis. J. Pediatr. Endocrinol. Metab. 19 (Suppl. 1), 321–324 (2006).
Riva, D. et al. Late neuropsychological and behavioural outcome of children surgically treated for craniopharyngioma. Childs Nerv. Syst. 14, 179–184 (1998).
Pierre-Kahn, A. et al. Social and psycho-intellectual outcome following radical removal of craniopharyngiomas in childhood. A prospective series. Childs Nerv. Syst. 21, 817–824 (2005).
Müller, H. L. et al. Longitudinal study on quality of life in 102 survivors of childhood craniopharyngioma. Childs Nerv. Syst. 21, 975–980 (2005).
Fisher, P. G. et al. Outcomes and failure patterns in childhood craniopharyngiomas. Childs Nerv. Syst. 14, 558–563 (1998).
DeVile, C. J., Grant, D. B., Hayward, R. D. & Stanhope, R. Growth and endocrine sequelae of craniopharyngioma. Arch. Dis. Child. 75, 108–114 (1996).
Merchant, T. E. et al. Radiation dose-volume effects on growth hormone secretion. Int. J. Radiat. Oncol. Biol. Phys. 52, 1264–1270 (2002).
Honegger, J., Buchfelder, M. & Fahlbusch, R. Surgical treatment of craniopharyngiomas: endocrinological results. J. Neurosurg. 90, 251–257 (1999).
Karavitaki, N. et al. GH replacement does not increase the risk of recurrence in patients with craniopharyngioma. Clin. Endocrinol. (Oxf.) 64, 556–560 (2006).
Lustig, R. H. et al. Risk factors for the development of obesity in children surviving brain tumours. J. Clin. Endocrinol. Metab. 88, 611–616 (2003).
Müller, H. L. et al. Obesity after childhood craniopharyngioma—German multicenter study on preoperative risk factors and quality of life. Klin. Padiatr. 213, 244–249 (2001).
Srinivasan, S. et al. Features of the metabolic syndrome after childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 89, 81–86 (2004).
Müller, H. L. et al. Volumetric bone mineral density in patients with childhood craniopharyngioma. Exp. Clin. Endocrinol. Diabetes 111, 168–173 (2003).
Müller, H. L. et al. Perioperative dexamethasone treatment in childhood craniopharyngioma—influence on short-term and long-term weight gain. Exp. Clin. Endocrinol. Diabetes 111, 330–334 (2003).
De Vile, C. J. et al. Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? J. Neurosurg. 85, 73–81 (1996).
Holmer, H. et al. Hypothalamic involvement predicts cardiovascular risk in adults with childhood onset craniopharyngioma on long-term GH therapy. Eur. J. Endocrinol. 161, 671–679 (2009).
Pereira, A. M. et al. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin. Endocrinol. (Oxf.) 62, 197–204 (2005).
de Vile, C. J. et al. Obesity in childhood craniopharyngioma: relation to post-operative hypothalamic damage shown by magnetic resonance imaging. J. Clin. Endocrinol. Metab. 81, 2734–2737 (1996).
Roth, C., Wilken, B., Hanefeld, F., Schröter, W. & Leonhardt, U. Hyperphagia in children with craniopharyngioma is associated with hyperleptinemia and a failure in the down regulation of appetite. Eur. J. Endocrinol. 138, 89–91 (1998).
Harz, K. J., Müller, H. L., Waldeck, E., Pudel, V. & Roth, C. Obesity in patients with craniopharyngioma: assessment of food intake and movement counts indicating physical activity. J. Clin. Endocrinol. Metab. 88, 5227–5231 (2003).
Müller, H. L., Handwerker, G., Wollny, B., Faldum, A. & Sörensen, N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 87, 3993–3996 (2002).
Müller, H. L. et al. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control 17, 583–589 (2006).
O'Gorman, C. S. et al. Sleep-disordered breathing is increased in obese adolescents with craniopharyngioma compared with obese controls. J. Clin. Endocrinol. Metab. 95, 2211–2218 (2010).
Müller, H. L. et al. Secondary narcolepsy may be an underrated cause of increased daytime sleepiness in obese patients after childhood craniopharyngioma. J. Pediatric Endocrinol. Metab. 19, 423–429 (2006).
Mason, P. W., Krawiecki, N. & Meacham, L. R. The use of dextroamphetamine to treat obesity and hyperphagia in children treated for craniopharyngioma. Arch. Pediatr. Adolesc. Med. 156, 887–892 (2002).
Lustig, R. H. et al. Octreotide therapy of pediatric hypothalamic obesity: a double-blind, placebo-controlled trial. J. Clin. Endocrinol. Metab. 88, 2586–2592 (2003).
Roth, C. L. et al. Reduced sympathetic metabolites in urine of obese patients with craniopharyngioma. Pediatr. Res. 61, 496–501 (2007).
Roth, C., Gebhardt, U. & Müller, H. L. Appetite-regulating hormone changes in patients with craniopharyngioma. Obesity doi: 10.1038/oby.2010.80.
Roth, C. L. et al. Changes of peripheral alpha-melanocyte-stimulating hormone in childhood obesity. Metabolism 59, 186–194 (2010).
Müller, H. L. et al. First experiences with laparoscopic gastric banding (LAGB) in the treatment of obese patients with childhood craniopharyngioma. Klin. Padiatr. 219, 323–325 (2007).
Lustig, R. H., Tsai, P., Hirose, S. & Farmer, D. L. Treatment of hypothalamic obesity by laparoscopic truncal vagotomy: early experience [abstract FC11-011]. Horm. Res. 72 (Suppl. 3), 48 (2009).
Inge, T. H. et al. Gastric bypass surgery for treatment of hypothalamic obesity after craniopharyngioma therapy. Nat. Clin. Pract. Endocrinol. Metab. 3, 606–609 (2007).
Schultes, B., Ernst, B., Schmid, F. & Thurnheer, M. Distal gastric bypass surgery for the treatment of hypothalamic obesity after childhood craniopharyngioma. Eur. J. Endocrinol. 161, 201–206 (2009).
Rottembourg, D. et al. Outcome after bariatric surgery in two adolescents with hypothalamic obesity following treatment of craniopharyngioma. J. Pediatr. Endocrinol. Metab. 22, 867–872 (2009).
Müller, H. L. et al. Functional capacity, obesity and hypothalamic involvement—cross-sectional study on 212 patients with childhood craniopharyngioma. Klin. Padiatr. 215, 310–314 (2003).
Müller, H. L. et al. Prognosis and sequela in patients with childhood craniopharyngioma—results of HIT-ENDO and update on KRANIOPHARYNGEOM 2000. Klin. Padiatr. 216, 343–348 (2004).
Dekkers, O. M. et al. Quality of life in treated adult craniopharyngioma patients. Eur. J. Endocrinol. 154, 483–489 (2006).
Sands, S. A. et al. Quality of life and behavioral follow-up study of pediatric survivors of craniopharyngioma. J. Neurosurg. 103 (Suppl. 4), 302–311 (2005).
Kendall-Taylor, P. et al. The clinical, metabolic and endocrine features and the quality of life in adults with childhood-onset craniopharyngioma compared with adult-onset craniopharyngioma. Eur. J. Endocrinol. 152, 557–567 (2005).
Müller, H. L. et al. Relapse pattern after complete resection and early progressions after incomplete resection of childhood craniopharyngioma. Klin. Padiatr. 218, 315–320 (2006)
Müller, H. L. et al. Current strategies in diagnostics and endocrine treatment of patients with childhood craniopharyngioma during follow-up – recommendations in KRANIOPHARYNGEOM 2000. Onkologie 28, 150–156 (2005).
Acknowledgements
H. L. Müller is supported by a grant of the Deutsche Kinderkrebsstiftung, Bonn, Germany.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Müller, H. Childhood craniopharyngioma—current concepts in diagnosis, therapy and follow-up. Nat Rev Endocrinol 6, 609–618 (2010). https://doi.org/10.1038/nrendo.2010.168
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2010.168
This article is cited by
-
Pediatric craniopharyngioma: a 20-year study on epidemiological features, clinical presentation, and survival outcomes in a tertiary care center from LMIC
Child's Nervous System (2024)
-
Vision-related quality of life in patients with childhood-onset craniopharyngioma
Scientific Reports (2023)
-
Pediatric craniopharyngiomas: magnetic resonance imaging assessment for hypothalamus-pituitary axis dysfunction and outcome prediction
Pediatric Radiology (2023)
-
Parafoveal and peripapillary vessel density in pediatric and juvenile craniopharyngioma patients
Scientific Reports (2022)
-
Exploring the pathological relationships between adamantinomatous craniopharyngioma and contiguous structures with tumor origin
Journal of Neuro-Oncology (2022)