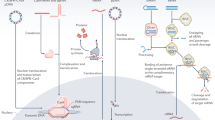
Key Points
-
Human gene therapy utilizes genetic material – DNA or RNA – as a therapeutic for genetic, acquired and infectious diseases. Gene therapy's potential is great, but the lack of safe and efficient gene delivery methods is a limiting obstacle to clinical implementation.
-
Gene delivery methods include recombinant viruses and synthetic materials such as lipids, polypeptides and polymers. Safety concerns limit the use of viral vectors. Non-viral gene delivery is typically much safer but suffers from generally unsatisfactory delivery efficiency.
-
Gene delivery vectors should protect the genetic material from nucleolytic enzymes, provide potentially long lifetime in the blood, and direct delivery to a specific tissue or cells. Furthermore, the vector must provide a mechanism for entering the target cell, transiting the cytosol, crossing the nuclear membrane and releasing the genetic material at the appropriate point in this process. Non-viral vectors typically lack one or more of the necessary functions.
-
The earliest synthetic vehicles reported in the literature are off-the-shelf materials, not originally designed for gene delivery. These include polylysine, polyethylenimine, and poly(amido amine) dendrimers. The gene delivery efficacy of such materials is serendipitous and, perhaps not surprisingly, typically insufficient for clinical application.
-
In recent years, a variety of polymers have been designed specifically for gene delivery. Often, such polymers are designed to be non-toxic and to address particular steps in the gene delivery process, for example, escape from endocytic vesicles into the cytoplasm. While many of these materials are better than off-the-shelf polymers, their delivery efficiency remains several orders of magnitude below that of recombinant viruses.
-
Based on the large number of studies of off-the-shelf and specifically designed gene delivery polymers, much has been learned about the structure-function relationships of polymer vectors. With growing understanding of polymer gene delivery mechanisms and continued efforts of creative and talented polymer chemists, it is likely that polymer-based gene delivery systems will become an important tool for human gene therapy.
Abstract
The lack of safe and efficient gene-delivery methods is a limiting obstacle to human gene therapy. Synthetic gene-delivery agents, although safer than recombinant viruses, generally do not possess the required efficacy. In recent years, a variety of effective polymers have been designed specifically for gene delivery, and much has been learned about their structure–function relationships. With the growing understanding of polymer gene-delivery mechanisms and continued efforts of creative polymer chemists, it is likely that polymer-based gene-delivery systems will become an important tool for human gene therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Mulligan, R. C. The basic science of gene therapy. Science 260, 926–932 (1993). An early review describing the key problems facing clinical implementation of human gene therapy.
Walsh, C. E. Gene therapy progress and prospects: gene therapy for the hemophilias. Gene Ther. 10, 999–1003 (2003).
van Deutekom, J. C. T. & van Ommen, G. J. B. Advances in Duchenne muscular dystrophy gene therapy. Nature Rev. Genet. 4, 774–783 (2003).
Ferrari, S., Geddes, D. M. & Alton, E. W. F. W. Barriers to and new approaches for gene therapy and gene delivery in cystic fibrosis. Adv. Drug Deliv. Rev. 54, 1373–1393 (2002).
Dzau, V. J., Deatt, K., Pompilio, G. & Smith, K. Current perceptions of cardiovascular gene therapy. Am. J. Cardiol. 92, 18–23 (2003).
Burton, E. A., Glorioso, J. C. & Fink, D. J. Gene therapy progress and prospects: Parkinson's disease. Gene Ther. 10, 1721–1727 (2003).
Alisky, J. M. & Davidson, B. L. Gene therapy for amyotrophic lateral sclerosis and other motor neuron diseases. Hum. Gene Ther. 11, 2315–2329 (2000).
Tuszynski, M. H. Growth-factor gene therapy for neurodegenerative disorders. Lancet Neurol. 1, 51–57 (2002).
Bunnell, B. A. & Morgan, R. A. Gene therapy for infectious diseases. Clin. Microbiol. Rev. 11, 42–52 (1998).
Cutroneo, K. R. Gene therapy for tissue regeneration. J. Cell. Biochem. 88, 418–425 (2003).
Vile, R. G., Russell, S. J. & Lemoine, N. R. Cancer gene therapy: hard lessons and new courses. Gene Ther. 7, 2–8 (2000).
Kerr, D. Clinical development of gene therapy for colorectal cancer. Nature Rev. Cancer 3, 615–622 (2003).
McNeish, L. A., Bell, S. J. & Lemoine, N. R. Gene therapy progress and prospects: cancer gene therapy using tumour suppressor genes. Gene Ther. 11, 497–503 (2004).
Nabel, E. G. Gene therapy for cardiovascular diseases. J. Nucl. Cardiol. 6, 69–75 (1999).
Liu, M. A. DNA vaccines: a review. J. Intern. Med. 253, 402–410 (2003).
Blaese, R. M. et al. T lymphocyte-directed gene therapy for ADA–SCID: initial trial results after 4 years. Science 270, 475–480 (1995).
Cavazzana-Calvo, M. et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288, 669–672 (2000). This paper reports the first 'successful' gene therapy trial in humans.
Kay, M. A. et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nature Genet. 24, 257–261 (2000).
Khuri, F. R. et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nature Med. 6, 879–885 (2000).
Gene Therapy Clinical Trials [online] <http://www.wiley.co.uk/genetherapy/clinical/> (2005). Website tabulating important statistics regarding gene therapy clinical trials, including their classification by disease and type of vector.
Verma, I. M. & Somia, N. Gene therapy — promises, problems and prospects. Nature 389, 239–242 (1997).
During, M. J. Adeno-associated virus as a gene delivery system. Adv. Drug Deliv. Rev. 27, 83–94 (1997).
Vile, R. G., Tuszynski, A. & Castleden, S. Retroviral vectors: from laboratory tools to molecular medicines. Mol. Biotechnol. 5, 139–158 (1996).
Felgner, P. L. et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl Acad. Sci. USA 84, 7413 (1987). This is the first report of gene delivery to mammalian cells using cationic lipids as the DNA carrier.
Koltover, I., Salditt, T., Radler, J. O. & Safinya, C. R. An inverted hexagonal phase of cationic liposome–DNA complexes related to DNA release and delivery. Science 281, 78–81 (1998). A key study that for the first time correlates the three-dimensional structures of lipoplexes with their transfection activity.
Zabner, J. Cationic lipids used in gene transfer. Adv. Drug Deliv. Rev. 27, 17–28 (1997).
Fillion, M. C. & Phillips, N. C. Major limitations in the use of cationic liposomes for DNA delivery. Int. J. Pharm. 162, 159–170 (1998).
Abdelhady, H. G. et al. Direct real-time molecular scale visualisation of the degradation of condensed DNA complexes exposed to DNase I. Nucleic Acids Res. 31, 4001–4005 (2003).
Bloomfield, V. A. DNA condensation by multivalent cations. Biopolymers 44, 269–282 (1997).
Wagner, E., Cotten, M., Foisner, R. & Birnstiel, M. L. Transferrin–polycation–DNA complexes: the effect of polycations on the structure of the complex and DNA delivery to cells. Proc. Natl Acad. Sci. USA 88, 4255–4259 (1991).
Hansma, H. G. et al. DNA condensation for gene therapy as monitored by atomic force microscopy. Nucleic Acids Res. 26, 2481–2487 (1998).
Lai, E. & van Zanten, J. H. Monitoring DNA/poly-L-lysine polyplex formation with time-resolved multiangle laser light scattering. Biophys. J. 80, 864–873 (2001).
Kabanov, A. V. et al. DNA interpolyelectrolyte complexes as a tool for efficient cell transformation. Biopolymers 31, 1437–1443 (1991).
Wadhwa, M. S., Collard, W. T., Adami, R. C., McKenzie, D. L. & Rice, K. G. Peptide-mediated gene delivery: influence of peptide structure on gene expression. Bioconjug. Chem. 8, 81–88 (1997).
Plank, C., Tang, M. X., Wolfe, A. R. & Szoka, F. C. Branched cationic peptides for gene delivery: role of type and number of cationic residues in formation and in vitro activity of DNA polyplexes. Hum. Gene Ther. 10, 319–332 (1999).
Schaffer, D. V., Fidelman, N. A., Dan, N. & Lauffenburger, D. A. Vector unpacking as a potential barrier for receptor-mediated polyplex gene delivery. Biotechnol. Bioeng. 67, 598–606 (2000). The first direct investigation of the effects of polymer–DNA interaction strength on gene-delivery efficiency.
Wolfert, M. A. et al. Polyelectrolyte vectors for gene delivery: influence of cationic polymer on biophysical properties of complexes formed with DNA. Bioconjug. Chem. 10, 993–1004 (1999).
Zelphati, O. & Szoka, F. C. Mechanism of oligonucleotide release from cationic liposomes. Proc. Natl Acad. Sci. USA 93, 11493–11498 (1996).
Dash, P. R., Read, M. L., Barrett, L. B., Wolfert, M. A. & Seymour, L. W. Factors affecting blood clearance and in vivo distribution of polyelectrolyte complexes for gene delivery. Gene Ther. 6, 643–650 (1999).
Ogris, M., Brunner, S., Schuller, S., Kircheis, R. & Wagner, E. PEGylated DNA/transferrin–PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 6, 595–605 (1999).
Toncheva, V. et al. Novel vectors for gene delivery formed by self-assembly of DNA with poly(L-lysine) grafted with hydrophilic polymers. Biochim. Biophys. Acta 1380, 354–368 (1998).
Oupicky, D. et al. Steric stabilization of poly-L-lysine/DNA complexes by the covalent attachment of semitelechelic poly[N-(2-hydroxypropyl)methacrylamide]. Bioconjug. Chem. 11, 492–501 (2000).
Dash, P. R. et al. Decreased binding to proteins and cells of polymeric gene delivery vectors surface modified with a multivalent hydrophilic polymer and retargeting through attachment of transferrin. J. Biol. Chem. 275, 3793–3802 (2000).
Howard, K. A. et al. Influence of cationic polymers on the biophysical properties of polyelectrolyte complexes formed by self-assembly with DNA. Biochim. Biophys. Acta 1475, 245–255 (2000).
Wang, W., Tetley, L. & Uchegbu, I. F. The level of hydrophobic substitution and the molecular weight of amphiphilic poly-L-lysine-based polymers strongly affects their assembly into polymeric bilayer vesicles. J. Colloid. Int. Sci. 237, 200–207 (2001).
Erbacher, P. et al. Gene transfer by DNA/glycosylated polylysine complexes into human blood monocyte-derived macrophages. Hum. Gene Ther. 7, 721–729 (1996).
Nishikawa, M., Takemura, S., Takakura, Y. & Hashida, M. Targeted delivery of plasmid DNA to hepatocytes in vivo optimization of the pharmacokinetics of plasmid DNA galactosylated poly(L-lysine) complexes by controlling their physicochemical properties. J. Pharm. Exp. Ther. 287, 408–415 (1998).
Kircheis, R. et al. Polyethylenimine/DNA complexes shielded by transferrin target gene expression to tumors after systemic application. Gene Ther. 8, 28–40 (2001).
Mishra, S., Webster, P. & Davis, M. E. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur. J. Cell Biol. 83, 97–111 (2004).
Erbacher, P., Roche, A. C., Monsigny, M. & Midoux, P. Glycosylated polylysine/DNA complexes: gene transfer efficiency in relation with the size and the sugar substitution level of glycosylated polylysines and with the plasmid size. Bioconjug. Chem. 6, 401–410 (1995).
Ferkol, T., Perales, J. C., Mularo, F. & Hanson, R. W. Receptor-mediated gene transfer into macrophages. Proc. Natl Acad. Sci. USA 93, 101–105 (1996).
Zanta, M. -A., Boussif, O., Adib, A. & Behr, J. -P. In vitro gene delivery to hepatocytes with galactosylated polyethylenimine. Bioconjug. Chem. 8, 839–844 (1997).
Bettinger, T., Remy, J. S. & Erbacher, P. Size reduction of galactosylated PEI/DNA complexes improves lectin-mediated gene transfer into hepatocytes. Bioconjug. Chem. 10, 558–561 (1999).
Leamon, C. P., Weigl, D. & Hendren, R. W. Folate copolymer-mediated transfection of cultured cells. Bioconjug. Chem. 10, 947–957 (1999).
Wu, G. Y. & Wu, C. H. Receptor-mediated in vitro gene transformation by a soluble DNA carrier system. J. Biol. Chem. 262, 4429–4432 (1987).
Wu, G. Y. & Wu, C. H. Receptor-mediated gene delivery and expression in vivo. J. Biol. Chem. 263, 14621–14624 (1988).
Wu, C. H., Wilson, J. M. & Wu, G. Y. Targeting genes: delivery and persistent expression of a foreign gene driven by mammalian regulatory elements in vivo. J. Biol. Chem. 264, 16985–16987 (1989).
Wu, G. Y. et al. Receptor-mediated gene delivery in vivo. J. Biol. Chem. 266, 14338–14342 (1991).
Cotten, M. et al. Transferrin-polycation-mediated introduction of DNA into human leukemic cells: stimulation by agents that affect the survival of transfected DNA or modulate transferrin receptor levels. Proc. Natl Acad. Sci. USA 87, 4033–4037 (1990). References 55–59 were the first reports of targeted gene delivery to specific cells using a polymer-based carrier.
Wagner, E., Zenke, M., Cotten, M., Beug, H. & Birnstiel, M. L. Transferrin-polycation conjugates as carriers for DNA uptake into cells. Proc. Natl Acad. Sci. USA 87, 3410–3414 (1990).
Zenke, M. et al. Receptor-mediated endocytosis of transferrin-polycation conjugates: an efficient way to introduce DNA into hematopoietic cells. Proc. Natl Acad. Sci. USA 87, 3655–3659 (1990).
Zatloukal, K. et al. Transferrinfection: a highly efficient way to express gene constructs in eukaryotic cells. Ann. NY Acad. Sci. 660, 136–153 (1992).
Schaffer, D. V., Neve, R. L. & Lauffenburger, D. A. Use of the green fluorescent protein as a quantitative reporter of epidermal growth factor receptor-mediated gene delivery. Tissue Eng. 3, 53–63 (1997).
Schaffer, D. V. in Chemical Engineering 125 (Massachusetts Institute of Technology, Cambridge, Massachusetts, 1998).
Kircheis, R. et al. Coupling of cell-binding ligands to polyethylenimine for targeted gene delivery. Gene Ther. 4, 409–418 (1997).
Harbottle, R. P. et al. An RGD-oligolysine peptide: a prototype construct for integrin-mediated gene delivery. Hum. Gene Ther. 9, 1037–1047 (1998).
Schaffer, D. V. & Lauffenburger, D. A. Optimization of cell surface binding enhances efficiency and specificity of molecular conjugate gene delivery. J. Biol. Chem. 273, 28004–28009 (1998).
Tseng, W. C., Haselton, F. R. & Giorgio, T. D. Transfection by cationic liposomes using simultaneous single cell measurements of plasmid delivery and transgene expression. J. Biol. Chem. 272, 25641–25647 (1997).
Mukherjee, S., Ghosh, R. N. & Maxfield, F. R. Endocytosis. Physiol. Rev. 77, 759–803 (1997).
Mislick, K. A. & Baldeschwieler, J. D. Evidence for the role of proteoglycans in cation-mediated gene transfer. Proc. Natl Acad. Sci. USA 93, 12349–12354 (1996).
Erbacher, P., Roche, A. C., Monsigny, M. & Midoux, P. Putative role of chloroquine in gene transfer into a human hepatoma cell line by DNA/lactosylated polylysine complexes. Exp. Cell Res. 225, 186–194 (1996).
Curiel, D. T., Agarwal, S., Wagner, E. & Cotten, M. Adenovirus enhancement of transferrin–polylysine-mediated gene delivery. Proc. Natl Acad. Sci. USA 88, 8850–8854 (1991).
Cristiano, R. J., Smith, L. c. & Woo, S. L. C. Hepatic gene therapy: adenovirus enhancement of receptor-mediated gene delivery and expression in primary hepatocytes. Proc. Natl Acad. Sci. USA 90, 2122–2126 (1993).
Wagner, E. et al. Coupling of adenovirus to transferrin–polylysine/DNA complexes greatly enhances receptor-mediated gene delivery and expression of transfected genes. Proc. Natl Acad. Sci. USA 89, 6099–6103 (1992).
Cotten, M. et al. High-efficiency receptor-mediated delivery of small and large (48 kilobase) gene constructs using the endosome-disruption activity of defective or chemically inactivated adenovirus particles. Proc. Natl Acad. Sci. USA 89, 6094–6098 (1992).
Wagner, E., Plank, C., Zatloukal, K., Cotten, M. & Birnstiel, M. L. Influenza virus hemagglutinin HA-2 N-terminal fusogenic peptides augment gene transfer by transferrin–polylysine–DNA complexes: toward a synthetic virus-like gene-transfer vehicle. Proc. Natl Acad. Sci. USA 89, 7934–7938 (1992).
Plank, C., Zatloukal, K., Cotten, M., Mechtler, K. & Wagner, E. Gene transfer into hepatocytes using asialoglycoprotein receptor mediated endocytosis of DNA complexed with an artificial tetra-antennary galactose ligand. Bioconjug. Chem. 3, 533–539 (1992).
Plank, C., Oberhauser, B., Mechtler, K., Koch, C. & Wagner, E. The influence of endosome-disruptive peptides on gene transfer using synthetic virus-like gene transfer systems. J. Biol. Chem. 269, 12918–12924 (1994).
Midoux, P. et al. Specific gene transfer mediated by lactosylated poly-L-lysine into hepatoma cells. Nucleic Acids Res. 21, 871–878 (1993).
Wyman, T. B. et al. Design, synthesis, and characterization of a cationic peptide that binds to nucleic acids and permeabilizes bilayers. Biochemistry 36, 3008–3017 (1997).
Lee, H., Jeong, J. H. & Park, T. G. A new gene delivery formulation of polyethylenimine/DNA complexes coated with PEG conjugated fusogenic peptide. J. Control. Release 76, 183–192 (2001).
Vaysse, L., Burgelin, I., Merlio, J. P. & Arveiler, B. Improved transfection using epithelial cell line-selected ligands and fusogenic peptides. Biochim. Biophys. Acta 1475, 369–376 (2000).
Luby-Phelps, K., Castle, P. E., Taylor, D. L. & Lanni, F. Hindered diffusion of inert tracer particles in the cytoplasm of mouse 3T3 cells. Proc. Natl Acad. Sci. USA 84, 4910–4913 (1987).
Lukacs, G. L. et al. Size-dependent DNA mobility in cytoplasm and nucleus. J. Biol. Chem. 275, 1625–1629 (2000). This is an important paper demonstrating that plasmid DNA is too large to diffuse through the cytosol and, as a result, some form of active transport or mixing must be responsible for transport of DNA to the nuclear membrane.
Lechardaeur, D. et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 6, 482–497 (1999).
Suh, J., Wirtz, D. & Hanes, J. Efficient active transport of gene nanocarriers to the cell nucleus. Proc. Natl Acad. Sci. USA 100, 3878–3882 (2003).
Subramanian, A., Ranganathan, P. & Diamond, S. L. Nuclear targeting peptide scaffolds for lipofection of nondividing mammalian cells. Nat. Biotechnol. 17, 873–877 (1999).
Brunner, S. et al. Cell cycle dependence of gene transfer by lipoplex, polyplex and recombinant adenovirus. Gene Ther. 7, 401–407 (2000).
Chan, C. K. & Jans, D. A. Enhancement of polylysine-mediated transferrinfection by nuclear localization sequences: polylysine does not function as a nuclear localization sequence. Hum. Gene Ther. 10, 1695–1702 (1999).
Chan, C. K., Senden, T. & Jans, D. A. Supramolecular structure and nuclear targeting efficiency determine the enhancement of transfection by modified polylysines. Gene Ther. 7, 1690–1697 (2000).
Sebestyén, M. G. et al. DNA vector chemistry: the covalent attachment of signal peptides to plasmid DNA. Nature Biotechnol. 16, 80–85 (1998).
Brandén, L. J., Mohamed, A. J. & Smith, C. I. E. A peptide nucleic acid-nuclear localization signal fusion that mediates nuclear transport of DNA. Nature Biotechnol. 17, 784–787 (1999).
Ciolina, C. et al. Coupling of nuclear localization signals to plasmid DNA and specific interaction of the conjugates with importin α. Bioconjug. Chem. 10, 46–55 (1999).
Bremner, K. H., Seymour, L. W., Logan, A. & Read, M. L. Factors influencing the ability of nuclear localization sequence peptides to enhance nonviral gene delivery. Bioconjug. Chem. 15, 152–161 (2004).
Wilson, G., Dean, B. S., Wang, G. & Dean, D. A. DNA vector chemistry: the covalent attachment of signal peptides to plasmid DNA. J. Biol. Chem. 16, 80–85 (1998).
Erbacher, P., Roche, A. C., Monsigny, M. & Midoux, P. The reduction of the positive charges of polylysine by partial gluconoylation increases the transfection efficiency of polylysine/DNA complexes. Biochim. Biophys. Acta 1324, 27–36 (1997).
Banaszczyk, M. G. et al. Poly-L-lysine-graft-PEG-comb-type polycation copolymers for gene delivery. J. Macromol. Sci. A 36, 1061–1084 (1999).
Zauner, W., Ogris, M. & Wagner, E. Polylysine-based transfection systems utilizing receptor-mediated delivery. Adv. Drug Deliv. Rev. 30, 97–113 (1998).
Cotten, M., Wagner, E. & Birnstiel, M. L. Receptor-mediated transport of DNA into eukaryotic cells. Meth. Enz. 217, 618–644 (1993).
Perales, J. C., Ferkol, T., Geegen, H., Ratnoff, O. D. & Hanson, R. W. Gene transfer in vivo: sustained expression and regulation of genes introduced into the liver by receptor-targeted uptake. Proc. Natl Acad. Sci. USA 91, 4086–4090 (1994).
Wadhwa, M. S., Knoell, D. L., Young, A. P. & Rice, K. G. Targeted gene delivery with a low molecular weight glycopeptide carrier. Bioconjug. Chem. 6, 283–291 (1995).
Hashida, M., Takemura, S., Nishikawa, M. & Takakura, Y. Targeted delivery of plasmid DNA complexed with galactosylated poly(L-lysine). Adv. Drug Deliv. Rev. 53, 301–310 (1998).
Suh, W., Chung, J. -K., Park, S. -H. & Kim, S. W. Anti-JL1 antibody-conjugated poly(L-lysine) for targeted gene delivery to leukemia T cells. J. Control. Release 72, 171–178 (2001).
Boussif, O. et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc. Natl Acad. Sci. USA 92, 7297–7301 (1995). The first report of polyethylenimine as a gene-delivery vector.
Diebold, S. S., Kursa, M., Wagner, E., Cotten, M. & Zenke, M. Mannose polyethylenimine conjugates for targeted DNA delivery into dendritic cells. J. Biol. Chem. 274, 19087–19094 (1999).
Kircheis, R., Blessing, R., Brunner, S., Wightman, L. & Wagner, E. Tumor targeting with surface-shielded ligand–polycation DNA complexes. J. Control. Release 72, 165–170 (2001).
Wojda, U. & Miller, J. L. Targeted transfer of polyethylenimine–avidin–DNA bioconjugates to hematopoietic cells using biotinylated monoclonal antibodies. J. Pharm. Sci. 89, 674–681 (2000).
Abdallah, B. et al. A powerful nonviral vector for in vivo gene transfer into the adult mammalian brain: polyethylenimine. Hum. Gene Ther. 7, 1947–1954 (1996).
Goula, D. et al. Size, diffusibility and transfection performance of linear PEI/DNA complexes in the mouse central nervous system. Gene Ther. 5, 712–717 (1998).
Boletta, A. et al. Nonviral gene delivery to the rat kidney with polyethylenimine. Hum. Gene Ther. 8, 1243–1251 (1997).
Goula, D. et al. Polyethylenimine-based intravenous delivery of transgenes to mouse lung. Gene Ther. 5, 1291–1295 (1998).
Ferrari, S. et al. Polyethylenimine shows properties of interest for cystic fibrosis gene therapy. Biochim. Biophys. Acta 1447, 219–225 (1999).
Coll, J. L. et al. In vivo delivery to tumors of DNA complexed with linear polyethylenimine. Hum. Gene Ther. 10, 1659–1666 (1999).
Behr, J. -P. The proton sponge: a trick to enter cells the viruses did not exploit. Chimia 51, 34–36 (1997). This review paper provides the first clear description of the proton-sponge hypothesis.
Suh, J., Paik, H. J. & Hwang, B. K. Ionization of polyethylenimine and polyallylamine at various pHs. Bioorg. Chem. 22, 318–327 (1994).
Forrest, M. L., Meister, G. E., Koerber, J. T. & Pack, D. W. Partial acetylation of polyethylenimine enhances in vitro gene delivery. Pharm. Res. 21, 365–371 (2004).
Thomas, M. & Klibanov, A. M. Enhancing polyethylenimine's delivery of plasmid DNA into mammalian cells. Proc. Natl Acad. Sci. USA 99, 14640–14645 (2002).
Tomalia, D. A., Naylor, A. M. & Goddard, W. A. Starburst cascade polymers: molecular-level control of size, shape, surface chemistry, topology, and flexibility from atoms to macroscopic matter. Angew. Chem. Int. Ed. Eng. 29, 138–175 (1990).
Haensler, J. & Szoka, F. C. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug. Chem. 4, 372–379 (1993).
Tanaka, S. et al. Targeted killing of carcinoembryonic antigen (CEA)-producing cholangiocarcinoma cells by polyamido amine dendrimer-mediated transfer of an Epstein-Barr virus (EBV)-based plasmid vector carrying the CEA promoter. Cancer Gene Ther. 7, 1241–1249 (2000).
Rudolph, C., Lausier, J., Naundorf, S., Muller, R. H. & Rosenecker, J. In vivo gene delivery to the lung using polyethylenimine and fractured polyamidoamine dendrimers. J. Gene Med. 2, 269–278 (2000).
Harada, Y. et al. Highly efficient suicide gene expression in hepatocellular carcinoma cells by Epstein–Barr virus-based plasmid vectors combined with polyamidoamine dendrimer. Cancer Gene Ther. 7, 27–36 (2000).
Marayuma-Tabata, H. et al. Effective suicide gene therapy in vivo by EBV-based plasmid vector coupled with polyamidoamine dendrimer. Gene Ther. 7, 53–60 (2000).
Tang, M. X., Redemann, C. T. & Szoka, F. C. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug. Chem. 7, 703–714 (1996).
Tang, M. X. & Szoka, F. C. The influence of polymer structure on the interactions of cationic polymers with DNA and morphology of the resulting complexes. Gene Ther. 4, 823–832 (1997).
Midoux, P., LeCam, E., Coulaud, D., Delain, E. & Pichon, C. Histidine containing peptides and polypeptides as nucleic acid vectors. Somat. Cell Mol. Genet. 27, 27–47 (2002).
Pack, D. W., Putnam, D. & Langer, R. Design of imidazole-containing endosomolytic biopolymers for gene delivery. Biotechnol. Bioeng. 67, 217–223 (2000).
Ihm, J. -E. et al. High transfection efficiency of poly(4-vinylimidazole) as a new gene carrier. Bioconjug. Chem. 14, 707–708 (2003).
Midoux, P. & Monsigny, M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjug. Chem. 10, 406–411 (1999).
Pichon, C., Roufai, M. B., Monsigny, M. & Midoux, P. Histidylated oligolysines increase the transmembrane passage and the biological activity of antisense oligonucleotides. Nucleic Acids Res. 28, 504–512 (2000).
Putnam, D., Gentry, C. A., Pack, D. W. & Langer, R. Polymer-based gene delivery with low cytotoxicity by a unique balance of side chain termini. Proc. Natl Acad. Sci. USA 98, 1200–1205 (2001).
Grimm, D. & Kay, M. A. From virus evolution to vector revolution: use of naturally occurring serotypes of adeno-associated virus (AAV) as novel vectors for human gene therapy. Curr. Gene Ther. 3, 281–304 (2003).
Skehel, J. & Wiley, D. C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 69, 531–569 (2000).
Ren, J., Sharpe, J. C., Collier, R. J., London, R. J. & Loneon, E. Membrane translocation of charged residues at the tips of hydrophobic helices in the T domain of diphtheria toxin. Biochemistry 38, 976–984 (1999).
Tolstikov, V. V., Cole, R., Fang, H. & Pincus, S. H. Influence of endosome-destabilizing peptides on efficacy of anti–HIV immunotoxins. Bioconjug. Chem. 8, 38–43 (1997).
Subbarao, N. K., Parente, R. A., Szoka, F. C., Nadasdi, L. & Pongraca, K. pH-dependent bilayer destabilization by an amphipathic peptide. Biochemistry 26, 2964–2972 (1987).
Parente, R. A., Nir, S. & Szoka, F. C. pH-dependent fusion of phosphatidylcholine small vesicles. J. Biol. Chem. 263, 4724–4730 (1988).
Stayton, P. S. et al. in Biomimetic Materials and Design: Biointerfacial Strategies, Tissue Engineering, and Targeted Drug Delivery (eds Dillow, A. K. & Lowman, A. M.) 471–506 (Marcel Dekker Inc., 2002).
Thomas, J. L. & Tirrell, D. A. Polyelectrolyte-sensitized phospholipid vesicles. Acc. Chem. Res. 25, 336–342 (1992).
Thomas, J. L., Barton, S. W. & Tirrell, D. A. Membrane solubilization by a hydrophobic polyelectrolyte: surface activity and membrane binding. Biophys. J. 67, 1101–1106 (1994).
Murthy, N., Robichaud, J. R., Tirrell, D. A., Stayton, P. S. & Hoffman, A. S. The design and synthesis of polymers for eukaryotic membrane disruption. J. Control. Release 61, 137–143 (1999).
Murthy, N., Chang, I., Stayton, P. S. & Hoffman, A. S. pH-sensitive hemolysis by random copolymers of alkyl acrylates and acrylic acid. Macromol. Symp. 172, 49–55 (2001).
Cheung, C. Y., Murthy, N., Stayton, P. S. & Hoffman, A. S. A pH-sensitive polymer that enhances cationic lipid-mediated gene transfer. Bioconjug. Chem. 12, 906–910 (2001).
Kyriakides, T. R. et al. pH-sensitive polymers that enhance intracellular drug delivery in vivo. J. Control. Release 78, 295–303 (2002).
Kiang, T. et al. Formulation of chitosan/DNA nanoparticles with poly(propylacrylic acid) enhances gene expression. J. Biomater. Sci. Polym. Ed. 15, 1405–1422 (2005).
Cheung, C. Y., Stayton, P. S. & Hoffman, A. S. Poly(propylacrylic acid)-mediated serum stabilization of cationic lipoplexes. J. Biomater. Sci. Polym. Ed. 16, 163–179 (2005).
Henry, S., Pirie, C., Stayton, P. S. & Hoffman, A. S. Membrane–disruption ability of maleic anhydride copolymers at endosomal pHs. Biomacromolecules (in the press).
Stephan, D. & Nabel, E. G. Gene and other biological therapies for vascular diseases. Fundam. Clin. Pharmacol. 11, 97–110 (1997).
Bulmus, V., Woodward, M., Lin, L., Stayton, P. S. & Hoffman, A. S. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J. Control. Release 93, 105–120 (2003).
El-Sayed, M., Hoffman, A. S. & Stayton, P. S. Influence of composition of novel pH-sensitive and glutathione-reactive polymeric carriers on their membrane-destabilizing activity. J. Control. Release (in the press).
Murthy, N., Campbell, J., Fausto, N., Hoffman, A. S. & Stayton, P. S. Bioinspired polymeric carriers than enhance intracellular delivery of biomolecular therapeutics. Bioconjug. Chem. 14, 412–419 (2003).
Murthy, N., Campbell, J., Fausto, N., Hoffman, A. S. & Stayton, P. S. Design and synthesis of pH-responsive polymeric carriers that target uptake and enhance the intracellular delivery of oligonucleotides to hepatocytes. J. Control. Release 89, 365–374 (2003).
Gonzalez, H., Hwang, S. & Davis, M. New class of polymers for the delivery of macromolecular therapeutics. Bioconjug. Chem. 10, 1068–1074 (1999). First report of a gene delivery polymer containing cyclodextrin as part of the polymer backbone.
Hwang, S. J., Bellocq, N. C. & Davis, M. E. Effects of structure of cyclodextrin-containing polymers on gene delivery. Bioconjug. Chem. 12, 280–290 (2001).
Reineke, T. M. & Davis, M. E. Structural effects of carbohydrate-containing polycations on gene delivery. 1. Carbohydrate size and its distance from change centers. Bioconjug. Chem. 14, 247–254 (2003).
Reineke, T. M. & Davis, M. E. Structural effects of carbohydrate-containing polycations on gene delivery. 2. Charge center type. Bioconjug. Chem. 14, 255–261 (2003).
Popielarski, S. R., Mishra, S. & Davis, M. E. Structural effects of carbohydrate-containing polycations on gene delivery. 3. cyclodextrin type and functionalization. Bioconjug. Chem. 14, 672–678 (2003).
Davis, M. E. et al. Self-assembling nucleic acid delivery vehicles via linear, water-soluble, cyclodextrin-containing polymers. Curr. Med. Chem. 11, 179–197 (2004).
Pun, S. H. & Davis, M. E. in Polymeric Gene Delivery (ed. Amiji, M.) 187–210 (CRC, Boca Raton; 2005).
Davis, M. E. & Brewster, M. E. Cyclodextrin-based pharmaceutics: past, present, and future. Nature Rev. Drug Discov. 3, 1023–1035 (2004).
Kihara, F., Arima, H., Tsutsumi, T., Hirayama, F. & Uekama, K. Effects of structure of polyamidoamine dendrimer on gene transfer efficiency of the dendrimer conjugate with α-cyclodextrin. Bioconjug. Chem. 13, 1211–1219 (2002).
Kihara, F., Arima, H., Tsutsumi, T., Hirayama, F. & Uekama, K. In vitro and in vivo gene transfer by an optimized α-cyclodextrin conjugate with polyamidoamine dendrimer. Bioconjug. Chem. 14, 342–350 (2003).
Arima, H., Kihara, F., Hirayama, F. & Uekama, K. Enhancement of gene expression by polyamidoamine dendrimer conjugates with α-, β-, and γ-cyclodextrins. Bioconjug. Chem. 12, 476–484 (2001).
Forrest, M. L., Gabrielson, N. & Pack, D. W. Cyclodextrin-polyethylenimine conjugates for targeted in vitro gene delivery. Biotechnol. Bioeng. 89, 416–423 (2005).
Pun, S. H. et al. Cyclodextrin-modified polyethylenimine polymers for gene delivery. Bioconjug. Chem. 15, 831–840 (2004).
Pun, S. H. & Davis, M. Development of a non-viral gene delivery vehicle for systemic application. Bioconjug. Chem. 13, 630–639 (2002).
Bellocq, N., Pun, S. & Davis, M. Transferrin-containing, cyclodextrin polymer-based particles for tumor-targeted gene delivery. Bioconjug. Chem. 14, 1122–1132 (2003).
Pun, S. et al. Biodistribution of RNA-cleaving DNA enzyme (DNAzyme) to tumor tissue by transferrin-modified, cyclodextrin-based particles. Cancer Biol. Ther. 3, 641–650 (2004).
Putnam, D. & Langer, R. Poly(4-hydroxy-L-proline ester): low-temperature polycondensation and plasmid DNA complexation. Macromolecules 32, 3568–3662 (1999).
Lim, Y. -B., Choi, Y. H. & Park, J. -S. A self-destroying polycationic polymer: biodegradable poly(4-hydroxy-L-proline ester). J. Am. Chem. Soc. 121, 5633–5639 (1999).
Lim, Y. -B., Kim, C. -H., Kim, K., Kim, S. W. & Park, J. -S. Development of a safe gene delivery system using biodegradable polymer, poly[α-(4-aminobutyl)-L-glycolic acid]. J. Am. Chem. Soc. 122, 6524–6525 (2000).
Lim, Y. -B. et al. Cationic hyperbranched poly(amino ester): a novel class of DNA condensing molecule with cationic surface, biodegradable three-dimensional structure, and tertiary amine groups in the interior. J. Am. Chem. Soc. 123, 2460–2461 (2001).
Koh, J. J. et al. Degradable polymeric carrier for the delivery of IL-10 plasmid DNA to prevent autoimmune insulitis of NOD mice. Gene Ther. 7, 2099–2104 (2000).
Lynn, D. M. & Langer, R. Degradable poly(β-amino esters): synthesis, characterization, and self-assembly with plasmid DNA. J. Am. Chem. Soc. 122, 10761–10768 (2000).
Lynn, D. M., Amiji, M. M. & Langer, R. pH-responsive polymer microspheres: rapid release of encapsulated material within the range of intracellular pH. Angew. Chem. Int. Ed. Eng. 40, 1707–1710 (2001).
Lynn, D. M., Anderson, D. G., Putnam, D. & Langer, R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. J. Am. Chem. Soc. 123, 8155–8156 (2001). One of the first reports of a combinatorial approach to synthesis of materials for gene delivery.
Little, S. R. et al. Poly-β amino ester-containing microparticles enhance the activity of nonviral genetic vaccines. Proc. Natl Acad. Sci. USA 101, 9534–9539 (2004).
Anderson, D. G. et al. A polymer library approach to suicide gene therapy for cancer. Proc. Natl Acad. Sci. USA 101, 16028–16033 (2004).
Forrest, M. L., Koerber, J. T. & Pack, D. W. A degradable polyethylenimine derivative with low toxicity for highly efficient gene delivery. Bioconjug. Chem. 14, 934–940 (2003).
Pichon, C. et al. Poly[Lys–(AEDTP)]: a cationic polymer that allows dissociation of pDNA/cationic polymer complexes in a reductive medium and enhances polyfection. Bioconjug. Chem. 13, 76–82 (2002).
Gosselin, M. A., Guo, W. & Lee, R. J. Efficient gene transfer using reversible cross-linked low molecular weight polyethylenimine. Bioconjug. Chem. 12, 989–994 (2001).
Sonawane, N. D., Szoka, R. C. & Verkman, A. S. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine–DNA polyplexes. J. Biol. Chem. 278, 44826–44831 (2003).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- LIPOPLEXES
-
Nanoparticles similar to polyplexes but formed by complexation of cationic lipids (or liposomes containing cationic lipids) and DNA.
- POLYPLEXES
-
Nanoparticles, typically ∼100 nm in diameter, formed by electrostatic complexation of cationic polymers (or polypeptides) and DNA.
- RECEPTOR-MEDIATED ENDOCYTOSIS
-
The process by which cells internalize various nutrients and signalling molecules. Binding of the ligand to a specific plasma membrane-bound receptor protein generally initiates the formation of clathrin-coated pits and subsequently formation of a vesicle (the endosome) inside the cell. Viruses, drugs and delivery vectors can also be internalized by displaying the natural ligand or an analogue.
- TRANSFECTION
-
Delivery of biomolecules, usually DNA or RNA, into mammalian cells in culture mediated by synthetic reagents such as polymers, polypeptides or lipids.
- ENDOLYSOSOMES
-
Cytoplasmic vacuoles formed by inward budding of the cell membrane during endocytosis. The organelles, first known as endosomes, acidify and fuse with enzyme-carrying vesicles from the Golgi to become lysosomes.
- IMPORTINS
-
Cytoplasmic proteins that bind nuclear localization signal peptides and, therefore, target proteins for transport across the nuclear membrane. Importins are recognized by the nuclear pore complex, a macromolecular assembly of more than 100 different proteins, and translocated (along with their cargo molecule) through the pore by an energy-dependent mechanism.
- INCLUSION COMPLEXES
-
Bi-molecular complexes in which the 'host' forms a cavity into which the 'guest' molecule binds through non-covalent (van der Waals) interactions.
- SUICIDE GENE
-
A gene that, once expressed in a target cell, causes death of the cell — for example, by initiating apoptosis or making the cell susceptible to the activity of a prodrug. One example of the latter is herpes simplex thymidine kinase, which activates drugs such as acyclovir and ganciclovir.
Rights and permissions
About this article
Cite this article
Pack, D., Hoffman, A., Pun, S. et al. Design and development of polymers for gene delivery. Nat Rev Drug Discov 4, 581–593 (2005). https://doi.org/10.1038/nrd1775
Issue Date:
DOI: https://doi.org/10.1038/nrd1775
This article is cited by
-
A potential paradigm in CRISPR/Cas systems delivery: at the crossroad of microalgal gene editing and algal-mediated nanoparticles
Journal of Nanobiotechnology (2023)
-
Emerging non-viral vectors for gene delivery
Journal of Nanobiotechnology (2023)
-
Molecular dynamics simulation of complexation between plasmid DNA and cationic peptides
Polymer Journal (2023)
-
Nanotechnology in stem cell research and therapy
Journal of Nanoparticle Research (2023)
-
Structure and Function of Cationic and Ionizable Lipids for Nucleic Acid Delivery
Pharmaceutical Research (2023)