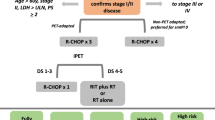
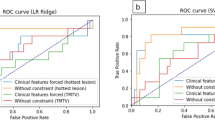
Abstract
The accurate detection and precise assessment of therapeutic responses is critical to the optimal management of patients with lymphoma. Over the past 50 years, dramatic advances in technology have established imaging as the cornerstone of disease evaluation. However, the appropriate application of current techniques requires acknowledgement of their strengths and weaknesses, and appreciation of the full diversity of lymphoid neoplasms. The role of anatomical and functional imaging in detection, treatment escalation/de-escalation and prognostication of patients with lymphoma can be misinterpreted. The development of disease assessment criteria, without an appreciation of the limitations of current imaging technologies, reflects a potential overreach of imaging science. Furthermore, the introduction of various novel therapies adds to the complexity of disease monitoring. In this Perspectives, the authors evaluate the available evidence in this rapidly evolving field and propose a reporting framework, named 'Specialist Integrated Haematological Malignancy Imaging Reporting' (SIHMIR), with a goal of providing a robust and adaptable system for lymphoma assessment. We predict a future model of multimodal disease assessment using novel molecular and imaging techniques, and highlight the key outstanding research questions in this field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Swerdlow, S. H. et al. The 2016 revision of the World Health Organization (WHO) classification of lymphoid neoplasms. Blood 127, 2375–2390 (2016).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2015. CA Cancer J. Clin. 65, 5–29 (2015).
Cheson, B. D. et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and Non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol. 32, 3059–3068 (2014).
National Institute for Health and Care Excellence. Non-Hodgkin's lymphoma: diagnosis management (NG52) [online]. https://www.nice.org.uk/guidance/ng52 (NICE, 2016).
National Institute for Health and Care Excellence. Myeloma: diagnosis management (NG35) [online]. https://www.nice.org.uk/guidance/ng35 (NICE, 2016).
National Institute for Health and Care Excellence. Haematological cancers: improving outcomes (NG47) [online]. https://www.nice.org.uk/guidance/ng47 (NICE, 2016).
Johnson, P. et al. Adapted treatment guided by interim PET-CT scan in advanced Hodgkin's lymphoma. N. Engl. J. Med. 374, 2419–2429 (2016).
Press, O. W. et al. US Intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose — positron emission tomography imaging: Southwest Oncology Group S0816. J. Clin. Oncol. 34, 2020–2027 (2016).
Rosenberg, S. A. Validity of the Ann Arbor staging classification for the non-Hodgkin's lymphomas. Cancer Treat. Rep. 61, 1023–1027 (1977).
Bernard, J. et al. Proposition d'une classification des different formes cliniques de la maladie de Hodgkin [French]. Nouv. Rev. Fr. Hematol. 6, 175–176 (1966).
Rosenberg, S. A. Report of the Committee on the Staging of Hodgkin's Disease. Cancer Res. 26, 1310 (1966).
Rosenberg, S. A. & Kaplan, H. S. Evidence for an orderly progression in the spread of Hodgkin's disease. Cancer Res. 26, 1225–1231 (1966).
Aisenberg, A. C. Primary management of Hodgkin's disease. CA Cancer J. Clin. 18, 158–162 (1968).
Musshoff, K. Prognostic and therapeutic implications of staging in extranodal Hodgkin's disease. Cancer Res. 31, 1814–1827 (1971).
Glatstein, E., Guernsey, J. M., Rosenberg, S. A. & Kaplan, H. S. The value of laparotomy and splenectomy in the staging of Hodgkin's disease. Cancer 24, 709–718 (1969).
Kadin, M. E., Glatstein, E. & Dorfman, R. F. Clinicopathologic studies of 117 untreated patients subjected to laparotomy for the staging of Hodgkin's Disease. Cancer 27, 1277–1294 (1971).
Strum, S. B. & Rappaport, H. Significance of focal involvement of lymph nodes for the diagnosis and staging of Hodgkin's disease. Cancer 25, 1314–1319 (1970).
Carbone, P. P., Kaplan, H. S., Musshoff, K., Smithers, D. W. & Tubiana, M. Report of the Committee on Hodgkin's Disease Staging Classification. Cancer Res. 31, 1860–1861 (1971).
Rosenberg, S. A. et al. Report of the Committee on Hodgkin's Disease Staging Procedures. Cancer Res. 31, 1862–1863 (1971).
Hounsfield, G. N. Computerized transverse axial scanning (tomography): part 1. Description of system. Br. J. Radiol. 46, 1016–1022 (1973).
Ambrose, J. Computerized transverse axial scanning (tomography): part 2. Clinical application. Br. J. Radiol. 46, 1023–1047 (1973).
Lister, T. A. et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J. Clin. Oncol. 7, 1630–1636 (1989).
Cheson, B. D. et al. Report of an international workshop to standardize response criteria for non-Hodgkin's lymphomas. J. Clin. Oncol. 17, 1244–1253 (1999).
Reivich, M. et al. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ. Res. 44, 127–137 (1979).
Warburg, O., Posener, K. & Negelein, E. Uber den Stoffwechsel der Carcinomzelle [German]. Biochem. Zeitschr. 152, 129–169 (1924).
Strauss, L. G. & Conti, P. S. The applications of PET in clinical oncology. J. Nucl. Med. 32, 623–648 (1991).
Rigo, P. et al. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. Eur. J. Nucl. Med. 23, 1641–1674 (1996).
Spaepen, K. et al. [18F]FDG PET monitoring of tumour response to chemotherapy: does [18F]FDG uptake correlate with the viable tumour cell fraction? Eur. J. Nucl. Med. Mol. Imaging 30, 682–688 (2003).
Gatenby, R. A. & Gillies, R. J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 (2004).
Spaepen, K., Stroobants, S., Verhoef, G. & Mortelmans, L. Positron emission tomography with [18F]FDG for therapy response monitoring in lymphoma patients. Eur. J. Nucl. Med. Mol. Imaging 30, S97–S105 (2003).
Isasi, C. R., Lu, P. & Blaufox, M. D. A metaanalysis of 18F-2-deoxy-2-fluoro-D-glucose positron emission tomography in the staging and restaging of patients with lymphoma. Cancer 104, 1066–1074 (2005).
Terasawa, T., Nihashi, T., Hotta, T. & Nagai, H. 18F-FDG PET for posttherapy assessment of Hodgkin's disease and aggressive non-Hodgkin's lymphoma: a systematic review. J. Nucl. Med. 49, 13–21 (2008).
Juweid, M. E. & Cheson, B. D. Role of positron emission tomography in lymphoma. J. Clin. Oncol. 23, 4577–4580 (2005).
Juweid, M. E. et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J. Clin. Oncol. 25, 571–578 (2007).
Juweid, M. E. & Cheson, B. D. Positron-emission tomography and assessment of cancer therapy. N. Engl. J. Med. 354, 496–507 (2006).
Beyer, T. et al. A combined PET/CT scanner for clinical oncology. J. Nucl. Med. 41, 1369–1379 (2000).
Cheson, B. D. et al. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 25, 579–586 (2007).
Parker, A. et al. Best Practice in Lymphoma Diagnosis and Reporting [online]. www.bloodmed.com/contentimage/guidelines/3455.pdf (2010).
Barrington, S. F. et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 32, 3048–3058 (2014).
Meignan, M., Gallamini, A. & Haioun, C. Report on the First International Workshop on interim-PET-scan in lymphoma. Leuk. Lymphoma 50, 1257–1260 (2009).
Eisenhauer, E. A. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45, 228–247 (2009).
Sharma, B., Martin, A., Stanway, S., Johnston, S. R. D. & Constantinidou, A. Imaging in oncology — over a century of advances. Nat. Rev. Clin. Oncol. 9, 728–737 (2012).
Castellino, R. A. et al. Computed tomography, lymphography, and staging laparotomy: correlations in initial staging of Hodgkin disease. AJR Am. J. Roentgenol. 143, 37–41 (1984).
Hasenclever, D. & Diehl, V. A. Prognostic score for advanced Hodgkin's disease. N. Engl. J. Med. 339, 1506–1514 (1998).
Josting, A. et al. New prognostic score based on treatment outcome of patients with relapsed Hodgkin's lymphoma registered in the database of the German Hodgkin's Lymphoma Study Group. J. Clin. Oncol. 20, 221–230 (2002).
Sehn, L. H. et al. The revised International Prognostic Index (R-IPI) is a better predictor of outcome than the standard IPI for patients with diffuse large B-cell lymphoma treated with R-CHOP. Blood 109, 1857–1861 (2007).
Hoster, E. et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 111, 558–565 (2008).
Federico, M. et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the International Follicular Lymphoma Prognostic Factor Project. J. Clin. Oncol. 27, 4555–4562 (2009).
Zhou, Z. et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood 123, 837–843 (2014).
Pastore, A. et al. Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry. Lancet Oncol. 16, 1111–1122 (2015).
Jurinovic, V. et al. Clinicogenetic risk models predict early progression of follicular lymphoma after first-line immunochemotherapy. Blood 128, 1112–1120 (2016).
Schmitz, N. et al. CNS International Prognostic Index: a risk model for CNS relapse in patients with diffuse large B-cell lymphoma treated with R-CHOP. J. Clin. Oncol. 34, 3150–3156 (2016).
Scherer, F. et al. Distinct biological subtypes and patterns of genome evolution in lymphoma revealed by circulating tumor DNA. Sci. Transl Med. 8, 364ra155 (2016).
Aukema, S. M. et al. Double-hit B-cell lymphomas. Blood 117, 2319–2331 (2011).
Cortelazzo, S. et al. Randomized trial comparing R-CHOP versus high-dose sequential chemotherapy in high-risk patients with diffuse large B-cell lymphomas. J. Clin. Oncol. 34, 4015–4022 (2016).
Schmitz, N. et al. Conventional chemotherapy (CHOEP-14) with rituximab or high-dose chemotherapy (MegaCHOEP) with rituximab for young, high-risk patients with aggressive B-cell lymphoma: an open-label, randomised, phase 3 trial (DSHNHL 2002–2001). Lancet Oncol. 13, 1250–1259 (2012).
Miller, T. P. et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin's lymphoma. N. Engl. J. Med. 339, 21–26 (1998).
Shenkier, T. N. et al. Brief chemotherapy and involved-region irradiation for limited-stage diffuse large-cell lymphoma: an 18-year experience from the British Columbia Cancer Agency. J. Clin. Oncol. 20, 197–204 (2002).
Leitch, H. A. et al. Limited-stage mantle-cell lymphoma. Ann. Oncol. 14, 1555–1561 (2003).
Vandenberghe, E. et al. The clinical outcome of 65 cases of mantle cell lymphoma initially treated with non-intensive therapy by the British National Lymphoma Investigation Group. Br. J. Haematol. 99, 842–847 (1997).
MacManus, M. P. & Hoppe, R. T. Is radiotherapy curative for stage I and II low-grade follicular lymphoma? Results of a long-term follow-up study of patients treated at Stanford University. J. Clin. Oncol. 14, 1282–1290 (1996).
Pugh, T. J., Ballonoff, A., Newman, F. & Rabinovitch, R. Improved survival in patients with early stage low-grade follicular lymphoma treated with radiation. Cancer 116, 3843–3851 (2010).
Engert, A. et al. Reduced treatment intensity in patients with early-stage Hodgkin's lymphoma. N. Engl. J. Med. 363, 640–652 (2010).
Noordijk, E. M. et al. Combined-modality therapy for clinical stage I or II Hodgkin's lymphoma: long-term results of the European Organisation for Research and Treatment of Cancer H7 randomized controlled trials. J. Clin. Oncol. 24, 3128–3135 (2006).
Carde, P. et al. Eight cycles of ABVD versus four cycles of BEACOPPescalated plus four cycles of BEACOPPbaseline in stage III to IV, International Prognostic Score ≥3, high-risk Hodgkin lymphoma: first results of the phase III EORTC 20012 Intergroup trial. J. Clin. Oncol. 34, 2028–2036 (2016).
Buchmann, I. et al. 2-(fluorine-18)fluoro-2-deoxy-D-glucose positron emission tomography in the detection and staging of malignant lymphoma: a bicenter trial. Cancer 91, 889–899 (2001).
Salaun, P. Y. et al. Analysis of 18F-FDG PET diffuse bone marrow uptake and splenic uptake in staging of Hodgkin's lymphoma: a reflection of disease infiltration or just inflammation? Eur. J. Nucl. Med. Mol. Imaging 36, 1813–1821 (2009).
Long, N. M. & Smith, C. S. Causes and imaging features of false positives and false negatives on 18F-PET/CT in oncologic imaging. Insights Imaging 2, 679–698 (2011).
Gawande, R. S. et al. Differentiation of normal thymus from anterior mediastinal lymphoma and lymphoma recurrence at pediatric PET/CT. Radiology 262, 613–622 (2012).
Jerushalmi, J., Frenkel, A., Bar-Shalom, R., Khoury, J. & Israel, O. Physiologic thymic uptake of 18F-FDG in children and young adults: a PET/CT evaluation of incidence, patterns, and relationship to treatment. J. Nucl. Med. 50, 849–853 (2009).
Dunleavy, K. et al. Dose-adjusted EPOCH-rituximab therapy in primary mediastinal B-cell lymphoma. N. Engl. J. Med. 368, 1408–1416 (2013).
Boellaard, R. et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur. J. Nucl. Med. Mol. Imaging 42, 328–354 (2015).
Martelli, M. et al. [18F]Fluorodeoxyglucose positron emission tomography predicts survival after chemoimmunotherapy for primary mediastinal large B-cell lymphoma: results of the International Extranodal Lymphoma Study Group IELSG-26 Study. J. Clin. Oncol. 32, 1769–1775 (2014).
Huttmann, A., Muller, S., Jockel, K.-H. & Duhrsen, U. Pitfalls of interim positron emission tomography scanning in diffuse large B-cell lymphoma. J. Clin. Oncol. 28, e488–e489 (2010).
Itti, E. et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur. J. Nucl. Med. Mol. Imaging 40, 1312–1320 (2013).
Hasenclever, D. et al. qPET — a quantitative extension of the Deauville scale to assess response in interim FDG-PET scans in lymphoma. Eur. J. Nucl. Med. Mol. Imaging 41, 1301–1308 (2014).
Le Roux, P. Y. et al. Prognostic value of interim FDG PET/CT in Hodgkin's lymphoma patients treated with interim response-adapted strategy: comparison of International Harmonization Project (IHP), Gallamini and London criteria. Eur. J. Nucl. Med. Mol. Imaging 38, 1064–1071 (2011).
Lim, E. et al. Guidelines on the radical management of patients with lung cancer. Thorax 65, iii1–iii27 (2010).
Kako, S. et al. FDG-PET in T-cell and NK-cell neoplasms. Ann. Oncol. 18, 1685–1690 (2007).
Perry, C. et al. Diagnostic accuracy of PET/CT in patients with extranodal marginal zone MALT lymphoma. Eur. J. Haematol. 79, 205–209 (2007).
Bodet-Milin, C. et al. Prognostic impact of 18F-fluoro-deoxyglucose positron emission tomography in untreated mantle cell lymphoma: a retrospective study from the GOELAMS group. Eur. J. Nucl. Med. Mol. Imaging 37, 1633–1642 (2010).
Friedberg, J. W. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am. Soc. Hematol. Educ. Program 2011, 498–505 (2011).
Engert, A. et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet 379, 1791–1799 (2012).
Dupuis, J. et al. Impact of [18F]fluorodeoxyglucose positron emission tomography response evaluation in patients with high-tumor burden follicular lymphoma treated with immunochemotherapy: a prospective study from the Groupe d'Etudes des Lymphomes de l'Adulte and GOELAMS. J. Clin. Oncol. 30, 4317–4322 (2012).
Gallamini, A. et al. Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin's lymphoma: a report from a joint Italian–Danish study. J. Clin. Oncol. 25, 3746–3752 (2007).
Moskowitz, C. H. et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J. Clin. Oncol. 28, 1896–1903 (2010).
Radford, J. et al. Results of a trial of PET-directed therapy for early-stage Hodgkin's lymphoma. N. Engl. J. Med. 372, 1598–1607 (2015).
Raemaekers, J. M. M. et al. Omitting radiotherapy in early positron emission tomography-negative stage I/II Hodgkin lymphoma is associated with an increased risk of early relapse: clinical results of the preplanned interim analysis of the randomized EORTC/LYSA/FIL H10 trial. J. Clin. Oncol. 32, 1188–1194 (2014).
Meyer, R. M. et al. ABVD alone versus radiation-based therapy in limited-stage Hodgkin's lymphoma. N. Engl. J. Med. 366, 399–408 (2012).
Schaapveld, M. et al. Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. N. Engl. J. Med. 373, 2499–2511 (2015).
Kumar, A. et al. Brentuximab vedotin and AVD followed by involved-site radiotherapy in early stage, unfavorable risk Hodgkin lymphoma. Blood 128, 1458–1464 (2016).
Barrington, S. F. & Mikhaeel, N. G. PET scans for staging and restaging in diffuse large B-cell and follicular lymphomas. Curr. Hematol. Malig. Rep. 11, 185–195 (2016).
Rapoport, A. P. et al. One hundred autotransplants for relapsed or refractory Hodgkin's disease and lymphoma: value of pretransplant disease status for predicting outcome. J. Clin. Oncol. 11, 2351–2361 (1993).
Jagannath, S. et al. Prognostic factors for response and survival after high-dose cyclophosphamide, carmustine, and etoposide with autologous bone marrow transplantation for relapsed Hodgkin's disease. J. Clin. Oncol. 7, 179–185 (1989).
Moskowitz, A. J. et al. Pretransplantation functional imaging predicts outcome following autologous stem cell transplantation for relapsed and refractory Hodgkin lymphoma. Blood 116, 4934–4937 (2010).
Moskowitz, C. H. Interim PET-CT in the management of diffuse large B-cell lymphoma. Hematology 2012, 397–401 (2012).
Moskowitz, C. H. et al. High-dose chemo-radiotherapy for relapsed or refractory Hodgkin lymphoma and the significance of pre-transplant functional imaging: research paper. Br. J. Haematol. 148, 890–897 (2010).
Cocorocchio, E. et al. High-dose chemotherapy in relapsed or refractory Hodgkin lymphoma patients: a reappraisal of prognostic factors. Hematol. Oncol. 31, 34–40 (2013).
Moskowitz, C. H. et al. Normalization of pre-ASCT, FDG-PET imaging with second-line, non-cross-resistant, chemotherapy programs improves event-free survival in patients with Hodgkin lymphoma. Blood 119, 1665–1670 (2012).
Moskowitz, A. J. et al. PET-adapted sequential salvage therapy with brentuximab vedotin followed by augmented ifosamide, carboplatin, and etoposide for patients with relapsed and refractory Hodgkin's lymphoma: a non-randomised, open-label, single-centre, phase 2 study. Lancet Oncol. 16, 284–292 (2015).
Derenzini, E. et al. Pretransplantation positron emission tomography scan is the main predictor of autologous stem cell transplantation outcome in aggressive B-cell non-Hodgkin lymphoma. Cancer 113, 2496–2503 (2008).
Dickinson, M. et al. Improved survival for relapsed diffuse large B cell lymphoma is predicted by a negative pre-transplant FDG-PET scan following salvage chemotherapy. Br. J. Haematol. 150, 39–45 (2010).
Hoppe, B. S. et al. The role of FDG-PET imaging and involved field radiotherapy in relapsed or refractory diffuse large B-cell lymphoma. Bone Marrow Transplant. 43, 941–948 (2009).
Sauter, C. S. et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood 125, 2579–2582 (2015).
Armand, P. et al. Prognostic factors for patients with diffuse large B cell lymphoma and transformed indolent lymphoma undergoing autologous stem cell transplantation in the positron emission tomography era. Br. J. Haematol. 160, 608–617 (2013).
Reyal, Y. et al. Impact of pretransplantation 18F-fluorodeoxyglucose-positron emission tomography on survival outcomes after T cell-depleted allogeneic transplantation for Hodgkin lymphoma. Biol. Blood Marrow Transplant. 22, 1234–1241 (2016).
Sauter, C. S. et al. Pretransplantation fluorine-18- deoxyglucose-positron emission tomography scan lacks prognostic value in chemosensitive B cell non-Hodgkin lymphoma patients undergoing nonmyeloablative allogeneic stem cell transplantation. Biol. Blood Marrow Transplant. 20, 881–903 (2014).
Bachanova, V. et al. Impact of pretransplantation 18F-fluorodeoxy glucose-positron emission tomography status on outcomes after allogeneic hematopoietic cell transplantation for non-Hodgkin lymphoma. Biol. Blood Marrow Transplant. 21, 1605–1611 (2015).
Armitage, J. O. Who benefits from surveillance imaging in lymphoma? J. Clin. Oncol. 30, 2579–2580 (2012).
Dann, E. J. et al. Hodgkin lymphoma patients in first remission: routine positron emission tomography/computerized tomography imaging is not superior to clinical follow-up for patients with no residual mass. Br. J. Haematol. 164, 694–700 (2014).
Thompson, C. A. et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J. Clin. Oncol. 32, 3506–3512 (2014).
Cohen, J. B., Behera, M., Thompson, C. A. & Flowers, C. R. Evaluating surveillance imaging for diffuse large B-cell lymphoma and Hodgkin lymphoma. Blood 129, 561–564 (2017).
Lambert, J. R. et al. Prognostic role of PET scanning before and after reduced-intensity allogeneic stem cell transplantation for lymphoma. Blood 115, 2763–2768 (2010).
Ulaner, G. A. et al. False-positive [18F]fluorodeoxyglucose-avid lymph nodes on positron emission tomography-computed tomography after allogeneic but not autologous stem-cell transplantation in patients with lymphoma. J. Clin. Oncol. 32, 51–56 (2013).
Therasse, P., Eisenhauer, E. A. & Verweij, J. RECIST revisited: a review of validation studies on tumour assessment. Eur. J. Cancer 42, 1031–1039 (2006).
Younes, A. et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann. Oncol. http://dx.doi.org/10.1093/annonc/mdx097 (2017).
Kumar, A. et al. Definition of bulky disease in early stage Hodgkin lymphoma in computed tomography era: prognostic significance of measurements in the coronal and transverse planes. Haematologica 101, 1237–1243 (2016).
Zasadny, K. R., Kison, P. V., Francis, I. R. & Wahl, R. L. FDG-PET determination of metabolically active tumor volume and comparison with CT. Clin. Positron Imaging 1, 123–129 (1998).
Larson, S. M. et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging: the visual response score and the change in total lesion glycolysis. Clin. Positron Imaging 2, 159–171 (1999).
Schöder, H. & Moskowitz, C. Metabolic tumor volume in lymphoma: hype or hope? J. Clin. Oncol. 34, 3591–3594 (2016).
Ng, A. K., LaCasce, A. & Travis, L. B. Long-term complications of lymphoma and its treatment. J. Clin. Oncol. 29, 1885–1892 (2011).
Lee, C. I., Haims, A. H., Monico, E. P., Brink, J. A. & Forman, H. P. Diagnostic CT scans: assessment of patient, physician, and radiologist awareness of radiation dose and possible risks. Radiology 231, 393–398 (2004).
National Council on Radiation Protection and Measurements. Ionizing radiation exposure of the population of the United States http://dx.doi.org/10.1017/CBO9781107415324.004 (NCRP, 2009).
Committee on Medical Aspects of Radiation in the Environment (COMARE). Patient radiation dose issues resulting from the use of CT in the UK (Public Health England, 2014).
Pierce, D. A. & Preston, D. L. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat. Res. 154, 178–186 (2000).
Cardis, E. et al. The 15-country collaborative study of cancer risk among radiation workers in the nuclear industry: estimates of radiation-related cancer risks. Radiat. Res. 167, 396–416 (2007).
Shrimpton, P. C., Jansen, J. T. M. & Harrison, J. D. Updated estimates of typical effective doses for common CT examinations in the UK following the 2011 national review. Br. J. Radiol. 89, 20150346 (2016).
Quinn, B., Dauer, Z., Pandit-Taskar, N., Schoder, H. & Dauer, L. T. Radiation dosimetry of 18F-FDG PET/CT: incorporating exam-specific parameters in dose estimates. BMC Med. Imaging 16, 41 (2016).
Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2 (National Research Council of the National Academies, 2006).
Brenner, D. J., Elliston, C. D., Hall, E. J. & Berdon, W. E. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am. J. Roentgenol. 176, 289–296 (2001).
Pearce, M. S. et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet 380, 499–505 (2012).
Hicks, L. K. et al. The ASH Choosing Wisely campaign: five hematologic tests and treatments to question. Blood 122, 3879–3883 (2013).
Howlader, N. et al. SEER Cancer Statistics Review, 1975–2013 (National Cancer Institute, 2015).
Lukes, R. J. Relationship of histologic features to clinical stages in Hodgkin's disease. Am. J. Roentgenol. Radium Ther. Nucl. Med. 90, 944–955 (1963).
Coppleson, L., Rappaport, H., Strum, S. & Rose, J. Analysis of the Rye classification of Hodgkin's disease. The prognostic significance of cellular composition. J. Natl Cancer Inst. 51, 379–390 (1973).
Richards, S. J. & Jack, A. S. The development of integrated haematopathology laboratories: a new approach to the diagnosis of leukaemia and lymphoma. Clin. Lab. Haematol. 25, 337–342 (2003).
Meignan, M. et al. Report on the Third International Workshop on Interim Positron Emission Tomography in Lymphoma held in Menton, France, 26–27 September 2011 and Menton 2011 consensus. Leuk. Lymphoma 53, 1876–1881 (2012).
Meignan, M. et al. Report on the 4th International Workshop on Positron Emission Tomography in Lymphoma held in Menton, France, 3–5 October 2012. Leuk. Lymphoma 55, 31–37 (2014).
Meignan, M. et al. Report on the 5th International Workshop on Positron Emission Tomography in Lymphoma held in Menton, France, 19–20 September 2014. Leuk. Lymphoma 56, 1229–1232 (2015).
Falchi, L. et al. Correlation between FDG/PET, histology, characteristics, and survival in 332 patients with chronic lymphoid leukemia. Blood 123, 2783–2791 (2014).
Arnold, C. W. et al. RadPath: a web-based system for integrating and correlating radiology and pathology findings during cancer diagnosis. Acad. Radiol. 23, 90–100 (2016).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N. Engl. J. Med. 372, 311–319 (2015).
Han, H. S., Escalón, M. P., Hsiao, B., Serafini, A. & Lossos, I. S. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin's lymphoma treated with rituximab-containing regimens. Ann. Oncol. 20, 309–318 (2009).
Chanan-Khan, A. et al. Tumor flare reaction associated with lenalidomide treatment in patients with chronic lymphocytic leukemia predicts clinical response. Cancer 117, 2127–2135 (2011).
Cheson, B. D. et al. Refinement of the Lugano classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 128, 2489–2496 (2016).
Cheson, B. D. et al. Novel targeted agents and the need to refine clinical end points in chronic lymphocytic leukemia. J. Clin. Oncol. 30, 2820–2822 (2012).
Byrd, J. C. et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N. Engl. J. Med. 371, 213–223 (2014).
Bottcher, S. et al. Minimal residual disease quantification is an independent predictor of progression-free and overall survival in chronic lymphocytic leukemia: a multivariate analysis from the randomized GCLLSG CLL8 trial. J. Clin. Oncol. 30, 980–988 (2012).
Thompson, P. A. & Wierda, W. G. Eliminating minimal residual disease as a therapeutic endpoint: working toward cure for patients with CLL. Blood 127, 1–30 (2015).
Galimberti, S. et al. Minimal residual disease after conventional treatment significantly impacts on progression-free survival of patients with follicular lymphoma: the FIL FOLL05 trial. Clin. Cancer Res. 20, 6398–6405 (2014).
Pott, C. et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: a European MCL Intergroup study. Blood 115, 3215–3223 (2010).
van der Velder, V. H. & van Dongen, J. J. MRD detection in acute lymphoblastic leukemia patients using Ig/TCR gene rearrangements as targets for real-time quantitative PCR. Methods Mol. Biol. 538, 115–150 (2009).
Böttcher, S. et al. Minimal residual disease detection in mantle cell lymphoma: methods and significance of four-color flow cytometry compared to consensus IGH-polymerase chain reaction at initial staging and for follow-up examinations. Haematologica 93, 551–559 (2008).
Pott, C. et al. MRD detection in B-cell non-Hodgkin lymphomas using Ig gene rearrangements and chromosomal translocations as targets for real-time quantitative PCR. Methods Mol. Biol. 971, 175–200 (2013).
Ladetto, M. et al. Next-generation sequencing and real-time quantitative PCR for minimal residual disease detection in B-cell disorders. Leukemia 28, 1299–1307 (2014).
Roschewski, M. et al. Circulating tumour DNA and CT monitoring in patients with untreated diffuse large B-cell lymphoma: a correlative biomarker study. Lancet Oncol. 16, 541–549 (2015).
Kurtz, D. M. et al. Noninvasive monitoring of diffuse large B-cell lymphoma by immunoglobulin high-throughput sequencing. Blood 125, 3679–3687 (2015).
Bohers, E. et al. Somatic mutations of cell-free circulating DNA detected by next-generation sequencing reflect the genetic changes in both germinal center B-cell-like and activated B-cell-like diffuse large B-cell lymphomas at the time of diagnosis. Haematologica 100, e280–e284 (2015).
Vandenberghe, P. et al. Non-invasive detection of genomic imbalances in Hodgkin/Reed-Sternberg cells in early and advanced stage Hodgkin's lymphoma by sequencing of circulating cell-free DNA: a technical proof-of-principle study. Lancet Haematol. 2, e55–e65 (2015).
Luminari, S. et al. Positron emission tomography response and minimal residual disease impact on progression-free survival in patients with follicular lymphoma. A subset analysis from the FOLL05 trial of the Fondazione Italiana Linfom. Haematologica 101, e66–e68 (2016).
Mansfield, P. & Maudsley, A. A. Medical imaging by NMR. Br. J. Radiol. 50, 188–194 (1977).
Takahara, T. et al. Diffusion weighted whole body imaging with background body signal suppression (DWIBS): technical improvement using free breathing, STIR and high resolution 3D display. Radiat. Med. 22, 275–282 (2004).
Punwani, S. et al. Quantitative diffusion weighted MRI: a functional biomarker of nodal disease in Hodgkin lymphoma? Cancer Biomark. 7, 249–259 (2010).
Mayerhoefer, M. E. et al. Evaluation of diffusion-weighted MRI for pretherapeutic assessment and staging of lymphoma: results of a prospective study in 140 patients. Clin. Cancer Res. 20, 2984–2993 (2014).
Mayerhoefer, M. E. et al. Evaluation of diffusion-weighted magnetic resonance imaging for follow-up and treatment response assessment of lymphoma: results of an 18F-FDG-PET/CT-controlled prospective study in 64 patients. Clin. Cancer Res. 21, 2506–2513 (2015).
Huang, M. Q. et al. Monitoring response to chemotherapy of non-Hodgkin's lymphoma xenografts by T2-weighted and diffusion-weighted MRI. NMR Biomed. 21, 1021–1029 (2008).
Chen, Y., Zhong, J., Wu, H. & Chen, N. The clinical application of whole-body diffusion-weighted imaging in the early assessment of chemotherapeutic effects in lymphoma: the initial experience. Magn. Reson. Imaging 30, 165–170 (2012).
Hagtvedt, T. et al. Diffusion-weighted MRI compared to FDG PET/CT for assessment of early treatment response in lymphoma. Acta Radiol. 56, 152–158 (2015).
Littooij, A. S. et al. Whole-body MRI-DWI for assessment of residual disease after completion of therapy in lymphoma: a prospective multicenter study. J. Magn. Reson. Imaging 42, 1646–1655 (2015).
Pichler, B. J., Judenhofer, M. S. & Wehrl, H. F. PET/MRI hybrid imaging: devices and initial results. Eur. Radiol. 18, 1077–1086 (2008).
Lishner, M. et al. Hematologic malignancies in pregnancy: management guidelines from an international consensus meeting. J. Clin. Oncol. 34, 501–508 (2016).
Shields, A. F. et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 4, 1334–1336 (1998).
Buck, A. K. et al. 3-deoxy-3-[18F]fluorothymidine-positron emission tomography for noninvasive assessment of proliferation in pulmonary nodules. Cancer Res. 62, 3331–3334 (2002).
Minamimoto, R. et al. Diffuse large B-cell lymphoma: prospective multicenter comparison of early interim FLT PET/CT versus FDG PET/CT with IHP, EORTC, Deauville, and PERCIST criteria for early therapeutic monitoring. Radiology 280, 220–229 (2016).
Schöder, H. et al. Prospective study of 3′-deoxy-3′-18F-fluorothymidine PET for early interim response assessment in advanced stage B-cell lymphoma. J. Nucl. Med. 57, 728–734 (2016).
Rice, S. L., Roney, C. A., Daumar, P. & Lewis, J. S. The next generation of positron emission tomography radiopharmaceuticals in oncology. Semin. Nucl. Med. 41, 265–282 (2011).
Lam, W. W. C. et al. Promising role of [18F] fluorocholine PET/CT versus [18F] fluorodeoxyglucose PET/CT in primary brain tumors — early experience. Clin. Neurol. Neurosurg. 113, 156–161 (2011).
Segal, E. et al. Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat. Biotechnol. 25, 675–680 (2007).
Colen, R. et al. NCI workshop report: clinical and computational requirements for correlating imaging phenotypes with genomics signatures. Transl Oncol. 7, 556–569 (2014).
Aerts, H. J. W. L. et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 5, 4006 (2014).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Tomita, N. et al. Central nervous system event in patients with diffuse large B-cell lymphoma in the rituximab era. Cancer Sci. 103, 245–251 (2012).
Fletcher, C. D. & Kahl, B. S. Central nervous system involvement in diffuse large B-cell lymphoma: an analysis of risks and prevention strategies in the post-rituximab era. Leuk. Lymphoma 55, 2228–2240 (2014).
McMillan, A. et al. Guideline on the prevention of secondary central nervous system lymphoma. Br. J. Haematol. 163, 168–181 (2013).
Hodgkin, T. On some morbid appearances of the absorbent glands and spleen. Med. Chir. Trans. 17, 68–114 (1832).
Wilks, S. Cases of enlargement of the lymphatic glands and spleen, (or Hodgkin's disease) with remarks. Guys Hosp. Rep. 11, 56–67 (1865).
Trousseau, A. in Lectures on Clinical Medicine 180–211 (The New Sydenham Society, 1868).
Anglesio, E. The Treatment of Hodgkin's Disease (Springer, 1969).
Röntgen, W. K. A new form of radiation. Science 3, 726–729 (1896).
Reed, D. M. On the pathological changes in Hodgkin's disease, with especial reference to its relationship to tuberculosis. Johns Hopkins Hosp. Rep. 10, 113–196 (1902).
Kinmonth, J. B. Lymphangiography in clinical surgery and particularly in the treatment of lymphoedema. Ann. R. Coll. Surg. Engl. 15, 300–315 (1954).
Hudack, S. S. & McMaster, P. D. The lymphatic participation in human cutaneous phenomena; a study of the minute lymphatics of the living skin. J. Exp. Med. 57, 751–774 (1933).
Gall, E. A. & Mallory, T. B. Malignant lymphoma: a clinico-pathologic survey of 618 cases. Am. J. Pathol. 18, 381–429 (1942).
Craver, L. F. Recent advances in treatment of lymphomas, leukemias and allied disorders. Bull. N. Y. Acad. Med. 24, 3–25 (1948).
Jackson, H. & Parker, F. Hodgkin's Disease and Allied Disorders (Oxford Univ. Press, 1947).
Peters, M. V. A study of survivals in Hodgkin's disease treated radiologically. Am. J. Roentgenol. Radium Ther. 63, 299–311 (1950).
Rappaport, H., Winter, W. & Hicks, E. Follicular lymphoma: a re-evaluation of its position in the scheme of malignant lymphoma based on a survey of 253 cases. Cancer 9, 792–814 (1956).
Rappaport, H. in Atlas of Tumor Pathology Section III, Fascicle 8 (Armed Forces Institute of Pathology, 1966).
Phelps, M. E., Hoffman, E. J., Mullani, N. A. & Ter-Pogossian, M. M. Application of annihilation coincidence detection to transaxial reconstruction tomography. J. Nucl. Med. 16, 210–224 (1975).
Lauterbur, P. C. Image formation by induced local interactions: examples employing nuclear magnetic resonance. Nature 242, 190–191 (1973).
Lukes, R. J. & Collins, R. D. Immunologic characterization of human malignant lymphomas. Cancer 34, 1488–1503 (1974).
Gerard-Marchant, R. et al. Classification of non-Hodgkin's lymphomas. Lancet 2, 406–408 (1974).
[No authors listed.] National Cancer Institute sponsored study of classifications of non-Hodgkin's lymphomas; summary and description of a working formulation for clinical usage. The Non-Hodgkin's Lymphoma Pathologic Classification Project. Cancer 49, 2112–2135 (1982).
Harris, N. L. et al. A revised European-American classification of lymphoid neoplasms: Blood 84, 1361–1392 (1994).
Jaffe, E. S., Harris, N. L., Stein, H. & Vardiman, J. W. Tumors of Haematopoietic and Lymphoid Tissues (IARC Press, 2001).
Swerdlow, S. H. et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues (IARC Press, 2008).
Acknowledgements
We are grateful to Hannah Holmes of the Royal Marsden Hospital for providing secretarial and research support, Isabel Castellano of the Royal Marsden Hospital for providing medical physics-related collaborative input, and to Barry Jenkins of the Institute of the Cancer Research Library Service for obtaining the original historical references from The British Library.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to researching data for the article, discussions of content, writing, and reviewing and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Cunningham, J., Iyengar, S. & Sharma, B. Evolution of lymphoma staging and response evaluation: current limitations and future directions. Nat Rev Clin Oncol 14, 631–645 (2017). https://doi.org/10.1038/nrclinonc.2017.78
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.78
This article is cited by
-
Role of interim positron emission tomography/computed tomography in assessment of lymphoma treatment response
Egyptian Journal of Radiology and Nuclear Medicine (2024)
-
Can pre-transplant 18F-choline positron emission tomography predict relapse following autologous stem cell transplantation in primary central nervous system lymphoma?
Bone Marrow Transplantation (2022)
-
Development and validation of a [18F]FDG PET/CT-based radiomics nomogram to predict the prognostic risk of pretreatment diffuse large B cell lymphoma patients
European Radiology (2022)
-
Role of diffusion MRI in diagnosis of mediastinal lymphoma: initial assessment and response to therapy
Egyptian Journal of Radiology and Nuclear Medicine (2021)
-
18F-FDG PET/CT imaging findings in anaplastic large cell lymphoma, a rare subtype of lymphoma
Cancer Imaging (2020)