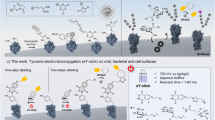
Abstract
We describe a protocol for the rapid labeling of cell-surface proteins in living mammalian cells using click chemistry. The labeling method is based on strain-promoted alkyne-azide cycloaddition (SPAAC) and strain-promoted inverse-electron–demand Diels–Alder cycloaddition (SPIEDAC) reactions, in which noncanonical amino acids (ncAAs) bearing ring-strained alkynes or alkenes react, respectively, with dyes containing azide or tetrazine groups. To introduce ncAAs site specifically into a protein of interest (POI), we use genetic code expansion technology. The protocol can be described as comprising two steps. In the first step, an Amber stop codon is introduced—by site-directed mutagenesis—at the desired site on the gene encoding the POI. This plasmid is then transfected into mammalian cells, along with another plasmid that encodes an aminoacyl-tRNA synthetase/tRNA (RS/tRNA) pair that is orthogonal to the host's translational machinery. In the presence of the ncAA, the orthogonal RS/tRNA pair specifically suppresses the Amber codon by incorporating the ncAA into the polypeptide chain of the POI. In the second step, the expressed POI is labeled with a suitably reactive dye derivative that is directly supplied to the growth medium. We provide a detailed protocol for using commercially available ncAAs and dyes for labeling the insulin receptor, and we discuss the optimal surface-labeling conditions and the limitations of labeling living mammalian cells. The protocol involves an initial cloning step that can take 4–7 d, followed by the described transfections and labeling reaction steps, which can take 3–4 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Glaeser, J., Nuss, A.M., Berghoff, B.A. & Klug, G. Singlet oxygen stress in microorganisms. Adv. Microb. Physiol. 58, 141–173 (2011).
Chudakov, D.M., Matz, M.V., Lukyanov, S. & Lukyanov, K.A. Fluorescent proteins and their applications in imaging living cells and tissues. Physiol. Rev. 90, 1103–1163 (2010).
Fernandez-Suarez, M. & Ting, A.Y. Fluorescent probes for super-resolution imaging in living cells. Nat. Rev. Mol. Cell Biol. 9, 929–943 (2008).
van de Linde, S., Heilemann, M. & Sauer, M. Live-cell super-resolution imaging with synthetic fluorophores. Annu. Rev. Phys. Chem. 63, 519–540 (2012).
Milles, S. & Lemke, E.A. What precision-protein-tuning and nano-resolved single molecule sciences can do for each other. BioEssays 35, 65–74 (2013).
Griffin, B.A., Adams, S.R. & Tsien, R.Y. Specific covalent labeling of recombinant protein molecules inside live cells. Science 281, 269–272 (1998).
Adams, S.R. et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: synthesis and biological applications. J. Am. Chem. Soc. 124, 6063–6076 (2002).
Keppler, A. et al. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat. Biotechnol. 21, 86–89 (2003).
Gautier, A. et al. An engineered protein tag for multiprotein labeling in living cells. Chem. Biol. 15, 128–136 (2008).
Los, G.V. & Wood, K. The HaloTag: a novel technology for cell imaging and protein analysis. Methods Mol. Biol. 356, 195–208 (2007).
Miller, L.W., Cai, Y., Sheetz, M.P. & Cornish, V.W. In vivo protein labeling with trimethoprim conjugates: a flexible chemical tag. Nat. Methods 2, 255–257 (2005).
Uttamapinant, C. et al. A fluorophore ligase for site-specific protein labeling inside living cells. Proc. Natl. Acad. Sci. USA 107, 10914–10919 (2010).
Uttamapinant, C. et al. Fast, cell-compatible click chemistry with copper-chelating azides for biomolecular labeling. Angew. Chem. Int. Ed. Engl. 51, 5852–5856 (2012).
Wang, L. & Schultz, P.G. Expanding the genetic code. Angew. Chem. Int. Ed. Engl. 44, 34–66 (2004).
Liu, C.C. & Schultz, P.G. Adding new chemistries to the genetic code. Annu. Rev. Biochem. 79, 413–444 (2010).
Lemke, E.A. The exploding genetic code. Chembiochem 15, 1691–1694 (2014).
Lang, K. & Chin, J.W. Cellular incorporation of unnatural amino acids and bioorthogonal labeling of proteins. Chem. Rev. 114, 4764–4806 (2014).
Wang, J., Xie, J. & Schultz, P.G. A genetically encoded fluorescent amino acid. J. Am. Chem. Soc. 128, 8738–8739 (2006).
Jewett, J.C. & Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 39, 1272–1279 (2010).
Agard, N.J., Prescher, J.A. & Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 126, 15046–15047 (2004).
Wiessler, M., Waldeck, W., Kliem, C., Pipkorn, R. & Braun, K. The Diels-Alder-reaction with inverse-electron-demand, a very efficient versatile click-reaction concept for proper ligation of variable molecular partners. Int. J. Med. Sci. 7, 19–28 (2009).
Blackman, M.L., Royzen, M. & Fox, J.M. Tetrazine ligation: fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc. 130, 13518–13519 (2008).
Kolb, H.C., Finn, M.G. & Sharpless, K.B. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. Engl. 40, 2004–2021 (2001).
Rostovtsev, V.V., Green, L.G., Fokin, V.V. & Sharpless, K.B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective 'ligation' of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 41, 2596–2599 (2002).
Plass, T., Milles, S., Koehler, C., Schultz, C. & Lemke, E.A. Genetically encoded copper-free click chemistry. Angew. Chem. Int. Ed. Engl. 50, 3878–3881 (2011).
Plass, T. et al. Amino acids for Diels-Alder reactions in living cells. Angew. Chem. Int. Ed. Engl. 51, 4166–4170 (2012).
Elliott, T.S. et al. Proteome labeling and protein identification in specific tissues and at specific developmental stages in an animal. Nat. Biotechnol. 32, 465–472 (2014).
Nikic, I. et al. Minimal tags for rapid dual-color live-cell labeling and super-resolution microscopy. Angew. Chem. Int. Ed. Engl. 53, 2245–2249 (2014).
Raulf, A. et al. Click chemistry facilitates direct labelling and super-resolution imaging of nucleic acids and proteins. RSC Adv. 4, 30462–30466 (2014).
Zhou, P. et al. Multicolor labeling of living-virus particles in live cells. Angew. Chem. Int. Ed. Engl. 51, 670–674 (2012).
Milles, S. et al. Click strategies for single-molecule protein fluorescence. J. Am. Chem. Soc. 134, 5187–5195 (2012).
Sun, S.B., Schultz, P.G. & Kim, C.H. Therapeutic applications of an expanded genetic code. Chembiochem 15, 1721–1729 (2014).
Patterson, D.M., Nazarova, L.A. & Prescher, J.A. Finding the right (bioorthogonal) chemistry. ACS Chem. Biol. 9, 592–605 (2014).
Liu, D.S. et al. Diels-Alder cycloaddition for fluorophore targeting to specific proteins inside living cells. J. Am. Chem. Soc. 134, 792–795 (2012).
Uttamapinant, C., Sanchez, M.I., Liu, D.S., Yao, J.Z. & Ting, A.Y. Site-specific protein labeling using PRIME and chelation-assisted click chemistry. Nat. Protoc. 8, 1620–1634 (2013).
Lee, H.S., Guo, J., Lemke, E.A., Dimla, R.D. & Schultz, P.G. Genetic incorporation of a small, environmentally sensitive, fluorescent probe into proteins in Saccharomyces cerevisiae. J. Am. Chem. Soc. 131, 12921–12923 (2009).
Summerer, D. et al. A genetically encoded fluorescent amino acid. Proc. Natl. Acad. Sci. USA 103, 9785–9789 (2006).
Herner, A. et al. New generation of bioorthogonally applicable fluorogenic dyes with visible excitations and large stokes shifts. Bioconjug. Chem. 25, 1370–1374 (2014).
Herner, A., Nikic, I., Kallay, M., Lemke, E.A. & Kele, P. A new family of bioorthogonally applicable fluorogenic labels. Org. Biomol. Chem. 11, 3297–3306 (2013).
Lukinavicius, G. et al. A near-infrared fluorophore for live-cell super-resolution microscopy of cellular proteins. Nat. Chem. 5, 132–139 (2013).
Meimetis, L.G., Carlson, J.C., Giedt, R.J., Kohler, R.H. & Weissleder, R. Ultrafluorogenic coumarin-tetrazine probes for real-time biological imaging. Angew. Chem. Int. Ed. Engl. 53, 7531–7534 (2014).
Lang, K. et al. Genetic encoding of bicyclononynes and trans-cyclooctenes for site-specific protein labeling in vitro and in live mammalian cells via rapid fluorogenic Diels-Alder reactions. J. Am. Soc. 134, 10317–10320 (2012).
Borrmann, A. et al. Genetic encoding of a bicyclo[6.1.0]nonyne-charged amino acid enables fast cellular protein imaging by metal-free ligation. Chembiochem 13, 2094–2099 (2012).
Parrish, A.R. et al. Expanding the genetic code of Caenorhabditis elegans using bacterial aminoacyl-tRNA synthetase/tRNA pairs. ACS Chem. Biol. 7, 1292–1302 (2012).
Greiss, S. & Chin, J.W. Expanding the genetic code of an animal. J. Am. Chem. Soc. 133, 14196–14199 (2011).
Carey, M.F., Peterson, C.L. & Smale, S.T. PCR-mediated site-directed mutagenesis. Cold Spring Harb. Protoc. 2013, 738–742 (2013).
Pott, M., Schmidt, M.J. & Summerer, D. Evolved sequence contexts for highly efficient amber suppression with noncanonical amino acids. ACS Chem. Biol. 9, 2815–2822 (2014).
Beier, H. & Grimm, M. Misreading of termination codons in eukaryotes by natural nonsense suppressor tRNAs. Nucleic Acids Res. 29, 4767–4782 (2001).
Phillips-Jones, M.K., Hill, L.S., Atkinson, J. & Martin, R. Context effects on misreading and suppression at UAG codons in human cells. Mol. Cell. Biol. 15, 6593–6600 (1995).
Yanagisawa, T. et al. Multistep engineering of pyrrolysyl-tRNA synthetase to genetically encode N(ɛ)-(o-azidobenzyloxycarbonyl) lysine for site-specific protein modification. Chem. Biol. 15, 1187–1197 (2008).
Mizuguchi, H., Xu, Z., Ishii-Watabe, A., Uchida, E. & Hayakawa, T. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol. Ther. 1, 376–382 (2000).
Ryan, M.D. & Drew, J. Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J. 13, 928–933 (1994).
Lang, K. et al. Genetically encoded norbornene directs site-specific cellular protein labelling via a rapid bioorthogonal reaction. Nat. Chem. 4, 298–304 (2012).
Sauer, J., Mielert, A., Lang, D. & Peter, D. Eine studie der Diels-Alder-reaktion, III: umsetzungen von 1.2.4.5-tetrazinen mit olefinen. Zur dtruktur von dihydropyridazinen. Chemische Berichte 98, 1435–1445 (1965).
Kaya, E. et al. A genetically encoded norbornene amino acid for the mild and selective modification of proteins in a copper-free click reaction. Angew. Chem. Int. Ed. Engl. 51, 4466–4469 (2012).
Li, J., Jia, S. & Chen, P.R. Diels-Alder reaction-triggered bioorthogonal protein decaging in living cells. Nat. Chem. Biol. 10, 1003–1005 (2014).
Acknowledgements
We thank the members of the Lemke and Schultz groups for proofreading and helpful discussions. We also thank SiChem for the gift of some of the compounds. This study was technically supported by the EMBL Advanced Light Microscopy Facility (ALMF) and the Proteomics Core Facility (PCF). I.N. acknowledges financial support by the European Commission Seventh Framework Programme (FP7) through Marie Curie Actions (FP7-PEOPLE-IEF) and a European Molecular Biology Organization (EMBO) long-term fellowship. E.A.L. acknowledges funding by the Emmy Noether program and SPP1623 of the Deutsche Forschungsgemeinschaft (DFG).
Author information
Authors and Affiliations
Contributions
I.N., J.H.K. and G.E.G. performed the experiments and conceived and wrote the protocol; I.V.A. performed validation experiments; and E.A.L. conceived and wrote the protocol.
Corresponding author
Ethics declarations
Competing interests
Some of the compounds used in this work are covered by a patent application.
Integrated supplementary information
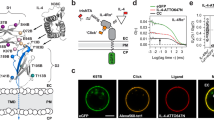
Supplementary Figure 2 Control strain-promoted alkyne-azide cycloaddition (SPAAC) labeling.
Unreactive non-canonical amino acid (BOC-Lys), IR(WT) or untransfected cells were labeled with Cy5-azide in the presence (a) and absence (b) of endocytosis blocker as done in main text Figure 3. Scale bar is 20μm.
Supplementary Figure 3 Strain-promoted alkyne-azide cycloaddition (SPAAC) short labeling.
Cells transfected with pIR(K676TAG)-GFP and expressing racBCN, endoBCN, exoBCN and SCO are labeled with 10 μM Cy5-azide dye for 10 min. Note that such labeling conditions (lower dye concentration and shorter incubation) did not lead to any noticeable labeling. Scale bar is 20μm.
Supplementary Figure 4 Control strain-promoted inverse electron-demand Diels-Alder cycloaddition (SPIEDAC) labeling with H-Tet-Cy5.
Unreactive non-canonical amino acid (BOC-Lys), IR(WT) or untransfected cells with H-Tet-Cy5. Labeling conditions were as in main text figure 4. Note that no labeling is observed. Scale bar is 20μm.
Supplementary Figure 5 Control strain-promoted inverse electron-demand Diels-Alder cycloaddition (SPIEDAC) labeling with Me-Tet-Cy5.
Unreactive non-canonical amino acid (BOC-Lys), IR(WT) or untransfected cells with Me-Tet-Cy5. Labeling conditions were as in main text figure 5. Scale bar is 20μm.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5, Supplementary Table 1 and Supplementary Methods (PDF 827 kb)
Rights and permissions
About this article
Cite this article
Nikić, I., Kang, J., Girona, G. et al. Labeling proteins on live mammalian cells using click chemistry. Nat Protoc 10, 780–791 (2015). https://doi.org/10.1038/nprot.2015.045
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2015.045
This article is cited by
-
A conformational switch in clathrin light chain regulates lattice structure and endocytosis at the plasma membrane of mammalian cells
Nature Communications (2023)
-
Minimal genetically encoded tags for fluorescent protein labeling in living neurons
Nature Communications (2022)
-
Bioorthogonal labeling of transmembrane proteins with non-canonical amino acids unveils masked epitopes in live neurons
Nature Communications (2021)
-
A far-red hybrid voltage indicator enabled by bioorthogonal engineering of rhodopsin on live neurons
Nature Chemistry (2021)
-
A straightforward approach for bioorthogonal labeling of proteins and organelles in live mammalian cells, using a short peptide tag
BMC Biology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.