Abstract
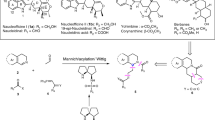
We describe two procedures for the synthesis of primary amines derived from 9-amino(9-deoxy)epi cinchona alkaloids, valuable catalysts used in the asymmetric functionalization of carbonyl compounds. The first approach allows the one-pot 5-g-scale syntheses of four cinchona-based analogs (1, 3, 5 and 7) from the alkaloids quinine (QN), quinidine (QD), dihydroquinine (DHQN) and dihydroquinidine (DHQD), respectively, performed by means of a Mitsunobu reaction to introduce an azide group, followed by reduction and hydrolysis. Demethylation of 1, 3, 5 and 7 with BBr3 provided direct access to the bifunctional aminocatalysts 2, 4, 6 and 8. A second approach, more convenient for scale-up (tested to a 20-g scale), is also provided. In this second procedure, the azides, formed from the O-mesylated derivatives of QN and QD, are selectively reduced with LiAlH4 to afford catalysts 1 and 3, whereas hydrogenation (Pd/C) provides 5 and 7. Demethylation of 1, 3, 5 and 7 using an alkylthiolate affords 2, 4, 6 and 8 in a process in which the less-expensive QN and QD are the only starting materials used.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
25 January 2013
In the version of this article initially published online, Fernando Bravo was not included as a corresponding author. Both Fernando Bravo and Paolo Melchiorre are corresponding authors for this manuscript. The error has been corrected in all versions of the article.
References
List, B. Emil Knoevenagel and the roots of aminocatalysis. Angew. Chem. Int. Ed. 49, 1730–1734 (2010).
Melchiorre, P., Marigo, M., Carlone, A. & Bartoli, G. Asymmetric aminocatalysis—gold rush in organic chemistry. Angew. Chem. Int. Ed. 47, 6138–6171 (2008).
Xu, L.-W., Luo, J. & Lu, Y. Asymmetric catalysis with chiral primary amine-based organocatalysts. Chem. Commun. 14, 1807–1821 (2009).
Jensen, K.L., Dickmeiss, G., Jiang, H., Albrecht, Ł. & Jørgensen, K.A. The diarylprolinol silyl ether system: a general organocatalyst. Acc. Chem. Res. 45, 248–264 (2011).
List, B. Proline-catalyzed asymmetric reactions. Tetrahedron 58, 5573–5590 (2002).
Lelais, G. & MacMillan, D.W.C. Modern strategies in organic catalysis: the advent and development of iminium activation. Aldrichimica Acta 39, 79–87 (2006).
List, B., Lerner, R.A. & Barbas, C.F. Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 122, 2395–2396 (2000).
Ahrendt, K.A., Borths, C.J. & MacMillan, D.W.C. New strategies for organic catalysis: the first highly enantioselective organocatalytic Diels-Alder reaction. J. Am. Chem. Soc. 122, 4243–4244 (2000).
Bertelsen, S., Marigo, M., Brandes, S., Dinér, P. & Jørgensen, K.A. Dienamine catalysis: organocatalytic asymmetric γ-amination of α,β-unsaturated aldehydes. J. Am. Chem. Soc. 128, 12973–12980 (2006).
Jia, Z.-J. et al. Trienamines in asymmetric organocatalysis: Diels-Alder and tandem reactions. J. Am. Chem. Soc. 133, 5053–5061 (2011).
Jiang, L. & Chen, Y.-C. Recent advances in asymmetric catalysis with cinchona alkaloid-based primary amines. Catal. Sci. Technol. 1, 354–365 (2011).
Bartoli, G. & Melchiorre, P. A novel organocatalytic tool for the iminium activation of α,β-unsaturated ketones. Synlett 1759–1772 (2008).
Sidorowicz, Ł. & Skarżewski, J. Easy access to 9-epimers of cinchona alkaloids: one-pot inversion by Mitsunobu esterification-saponification. Synthesis 708–710 (2011).
He, W., Liu, P., Zhang, B.L., Sun, X.L. & Zhang, S.Y. Efficient iridium and rhodium-catalyzed asymmetric transfer hydrogenation using 9-amino (9-deoxy) cinchona alkaloids as chiral ligands. Appl. Organomet. Chem. 20, 328–334 (2006).
Brunner, H., Bugler, J. & Nuber, B. Enantioselective catalysis 98. Preparation of 9-amino-(9-deoxy)cinchona alkaloids. Tetrahedron Asymmetry 6, 1699–1702 (1995).
Sundermeier, U., Döbler, C., Mehltretter, G.M., Baumann, W. & Beller, M. Synthesis of 9-N-cinchona alkaloid peptide hybrid derivatives: preparation and conformational study of 9-N-acylamino(9-deoxy)cinchona alkaloids. Chirality 15, 127–134 (2003).
Vakulya, B., Varga, S., Csampai, A. & Soos, T. Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional cinchona organocatalysts. Org. Lett. 7, 1967–1969 (2005).
He, W., Zhang, B., Liu, P., Sun, X. & Zhang, S. Synthesis of chiral diamine ligands derived from cinchona alkaloids and their catalytic performance for asymmetric transfer hydrogenation. Chin. J. Catal. 27, 527–531 (2006).
McCooey, S.H. & Connon, S.J. Readily accessible 9-epi-amino cinchona alkaloid derivatives promote efficient, highly enantioselective additions of aldehydes and ketones to nitroolefins. Org. Lett. 9, 599–602 (2007).
Melchiorre, P. Cinchona-based primary amine catalysis in the asymmetric functionalization of carbonyl compounds. Angew. Chem. Int. Ed. 51, 9748–9770 (2012).
Bartoli, G. et al. Organocatalytic asymmetric Friedel-Crafts alkylation of indoles with simple α,β-unsaturated ketones. Org. Lett. 9, 1403–1405 (2007).
Lu, X. & Deng, L. Asymmetric aza-Michael reactions of α,β-unsaturated ketones with bifunctional organic catalysts. Angew. Chem. Int. Ed. 47, 7710–7713 (2008).
Lu, X., Liu, Y., Sun, B., Cindric, B. & Deng, L. Catalytic enantioselective peroxidation of α,β-unsaturated ketones. J. Am. Chem. Soc. 130, 8134–8135 (2008).
Galzerano, P., Pesciaioli, F., Mazzanti, A., Bartoli, G. & Melchiorre, P. Asymmetric organocatalytic cascade reactions with α-substituted α,β-unsaturated aldehydes. Angew. Chem. Int. Ed. 48, 7892–7894 (2009).
Pesciaioli, F. et al. Organocatalytic asymmetric aziridination of enones. Angew. Chem. Int. Ed. 47, 8703–8706 (2008).
De Vincentiis, F et al. Asymmetric catalytic aziridination of cyclic enones. Chem. Asian J. 5, 1652–1656 (2010).
Zhang, E., Fan, C.-A., Tu, Y.-Q., Zhang, F.-M. & Song, Y.-L. Organocatalytic asymmetric vinylogous α-ketol rearrangement: enantioselective construction of chiral all-carbon quaternary stereocenters in spirocyclic diketones via semipinacol-type 1,2-carbon migration. J. Am. Chem. Soc. 131, 14626–14627 (2009).
Lifchits, O., Reisinger, C.M. & List, B. Catalytic asymmetric epoxidation of α-branched enals. J. Am. Chem. Soc. 132, 10227–10229 (2010).
Lv, J., Zhang, J., Lin, Z. & Wang, Y. Enantioselective synthesis of functionalized nitrocyclopropanes by organocatalytic conjugate addition of bromonitroalkanes to α,β-unsaturated enones. Chem. Eur. J. 15, 972–979 (2009).
Zhou, J., Wakchaure, V., Kraft, P. & List, B. Primary-amine-catalyzed enantioselective intramolecular aldolizations. Angew. Chem. Int. Ed. 47, 7656–7658 (2008).
Bencivenni, G. et al. Targeting structural and stereochemical complexity by organocascade catalysis: construction of spirocyclic oxindoles having multiple stereocenters. Angew. Chem. Int. Ed. 48, 7200–7203 (2009).
Wu, L.-Y. et al. Organocascade reactions of enones catalyzed by a chiral primary amine. Angew. Chem. Int. Ed. 48, 7196–7199 (2009).
Kwiatkowski, P., Beeson, T.D., Conrad, J.C. & MacMillan, D.W.C. Enantioselective organocatalytic α-fluorination of cyclic ketones. J. Am. Chem. Soc. 133, 1738–1741 (2011).
Bencivenni, G., Galzerano, P., Mazzanti, A., Bartoli, G. & Melchiorre, P. Direct asymmetric vinylogous Michael addition of cyclic enones to nitroalkenes via dienamine catalysis. Proc. Natl. Acad. Sci. USA 107, 20642–20647 (2010).
Bergonzini, G., Vera, S. & Melchiorre, P. Cooperative organocatalysis for the asymmetric γ alkylation of α-branched enals. Angew. Chem. Int. Ed. 49, 9685–9688 (2010).
Xiong, X.-F. et al. Trienamine catalysis with 2,4-dienones: development and application in asymmetric Diels-Alder reactions. Angew. Chem. Int. Ed. 51, 4401–4404 (2012).
Kotke, M. & Schreiner, P.R. (Thio)urea organocatalysts. in Hydrogen Bonding in Organic Synthesis (ed. Pihko, P.M.) 141–351 (Wiley-VCH, 2009).
Connon, S.J. Asymmetric catalysis with bifunctional cinchona alkaloid-based urea and thiourea organocatalysts. Chem. Commun. 14, 2499–2510 (2008).
Tian, X. et al. Diastereodivergent asymmetric sulfa-Michael additions of α-branched enones using a single chiral organic catalyst. J. Am. Chem. Soc. 133, 17934–17941 (2011).
Dijkstra, G.D.H. et al. Conformational study of cinchona alkaloids. A combined NMR, molecular mechanics and X-ray approach. J. Am. Chem. Soc. 111, 8069–8076 (1989).
Mitsunobu, O. The use of diethyl azodicarboxylate and triphenylphosphine in synthesis and transformation of natural products. Synthesis 1–28 (1981).
Swamy, K.C.K., Kumar, N.N.B., Balaraman, E. & Kumar, K.V.P.P. Mitsunobu and related reactions: advances and applications. Chem. Rev. 109, 2551–2651 (2009).
Simon, C., Hosztafi, S. & Makleit, S. Application of the Mitsunobu reaction in the field of alkaloids. J. Heterocyclic Chem. 34, 349–365 (1997).
Staudinger, H. & Meyer, J. Ueber neue organische Phosphorverbindungen II. Phosphazine. Helv. Chim. Acta 2, 619–635 (1919).
Wang, Y., Liu, X. & Deng, L. Dual-function cinchona alkaloid catalysis: catalytic asymmetric tandem conjugate additionprotonation for the direct creation of nonadjacent stereocenters. J. Am. Chem. Soc. 128, 3928–3930 (2006).
Chen, W. et al. Enantioselective 1,3-dipolar cycloaddition of cyclic enones catalyzed by multifunctional primary amines: Beneficial effects of hydrogen bonding. Angew. Chem. Int. Ed. 46, 7667–7670 (2007).
Trost, B. The atom economy-a search for synthetic efficiency. Science 254, 1471–1477 (1991).
Kucerovy, A., Li, T., Prasad, K., Repič, O. & Blacklock, T.J. An efficient large-scale synthesis of methyl 5-[2-(2,5-dimethoxyphenyl)ethyl]-2-hydroxybenzoate. Org. Process Res. Dev. 1, 287–293 (1997).
Ainge, D., Ennis, D., Gidlund, M., Stefinovic, M. & Vaz, L.-M. Rapid development of an enantioselective synthesis of (R)-1-hydroxy-7-methoxy-1,2,3,4-tetrahydronaphthalene-1-carboxylic acid. Org. Process Res. Dev. 7, 198–201 (2003).
Königsberger, K. et al. A practical synthesis of 6-[2-(2,5-dimethoxyphenyl)ethyl]-4-ethylquinazoline and the art of removing palladium from the products of Pd-catalyzed reactions. Org. Process Res. Dev. 7, 733–742 (2003).
Franz, M.H., Röper, S., Wartchow, R. & Hoffmann, H.M.R. The first and second cinchona rearrangement. two fundamental transformations of alkaloid chemistry. J. Org. Chem. 69, 2983–2991 (2004).
Kacprzak, K. & Gierczyk, B. Clickable 9-azido-(9-deoxy)-cinchona alkaloids: synthesis and conformation. Tetrahedron Asymmetry 21, 2740–2745 (2010).
Enders, D. & Müller-Hüwen, A. Asymmetric synthesis of 2-amino-1,3-diols and D-erythro-Sphinganine. Eur. J. Org. Chem 2004, 1732–1739 (2004).
Jeong, J.W. et al. A substrate mimetic approach for influenza neuraminidase inhibitors. Bull. Korean Chem. Soc. 25, 1575–1577 (2004).
Busscher, G.F., van den Broek, S.A.M.W., Rutjes, F.P.J.T. & van Delft, F.L. Carbohydrate mimic of 2-deoxystreptamine for the preparation of conformationally constrained aminoglycosides. Tetrahedron 63, 3183–3188 (2007).
Neumann, J. & Thiem, J. Synthesis of amino-bridged oligosaccharide mimetics. Eur. J. Org. Chem. 2010, 900–908 (2010).
Chae, J. Practical demethylation of aryl methyl ethers using an odorless thiol reagent. Arch. Pharm. Res. 31, 305–309 (2008).
Xu, F. et al. Asymmetric synthesis of a potent, aminopiperidine-fused imidazopyridine dipeptidyl peptidase IV inhibitor. J. Org. Chem. 75, 1343–1353 (2010).
Bräse, S., Gil, C., Knepper, K. & Zimmermann, V. Organic azides: an exploding diversity of a unique class of compounds. Angew. Chem. Int. Ed. 44, 5188–5240 (2005).
Nielsen, M.A., Nielsen, M.K. & Pittelkow, T. Scale-up and safety evaluation of a Sandmeyer reaction. Org. Process Res. Dev. 8, 1059–1064 (2004).
Mickel, S.J. et al. Large-scale synthesis of the anti-cancer marine natural product (+)-discodermolide. Part 1: Synthetic strategy and preparation of a common precursor. Org. Process Res. Dev. 8, 92–100 (2003).
Kopach, M.E., Murray, M.M., Braden, T.M., Kobierski, M.E. & Williams, O.L. Improved synthesis of 1-(azidomethyl)-3,5-bis-(trifluoromethyl)benzene: development of batch and microflow azide processes. Org. Process Res. Dev. 13, 152–160 (2009).
Scott, J.P. et al. Mitsunobu inversion of a secondary alcohol with diphenylphosphoryl azide. Application to the enantioselective multikilogram synthesis of a HCV polymerase inhibitor. Org. Process Res. Dev. 15, 1116–1123 (2011).
Conrow, R.E. & Dean, W.D. Diazidomethane explosion. Org. Process Res. Dev. 12, 1285–1286 (2008).
am Ende, D.J. & Vogt, P.F. Safety notables: information from the literature. Org. Process Res. Dev. 8, 1045–1048 (2004).
Gálvez, N., Moreno-Mañas, M., Sebastián, R.M. & Vallribera, A. Dimethoxyethane as an alternative solvent for Schmidt reactions. Preparation of homochiral N-(5-oxazolyl)oxazolidinones from N-acetoacetyl derivatives of oxazolidinones. Tetrahedron 52, 1609–1616 (1996).
Acknowledgements
We thank the ICIQ's NMR service for low-temperature 13C NMR, the ICIQ's Chemical Reaction Technologies Unit for technical support in performing pressurized hydrogenation reactions and the ICIQ's Chromatography, Thermal Analysis and Electrochemistry Unit for technical support in DSC analysis. Research support from the ICIQ Foundation, Ministerio de Ciencia e Innovación (MICINN) (grant no. CTQ2010-15513 and A Formación de Personal Investigador (FPI) predoctoral fellowship to C.C.) and the European Research Council (ERC starting grant agreement no. 278541 – ORGA-NAUT) is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
Synthetic work was carried out by C.C., R.M.-R. and F.B.; experimental procedure development and assembly of the manuscript was performed by C.C., R.M.-R., E.A., F.B. and P.M.
Corresponding authors
Ethics declarations
Competing interests
F.B., R.M-R. and P.M. have filed a patent application describing Approach 2 discussed in this protocol (application number EP12382291).
Supplementary information
Supplementary Data
1H NMR and 13C spectra of the products (PDF 1376 kb)
Rights and permissions
About this article
Cite this article
Cassani, C., Martín-Rapún, R., Arceo, E. et al. Synthesis of 9-amino(9-deoxy)epi cinchona alkaloids, general chiral organocatalysts for the stereoselective functionalization of carbonyl compounds. Nat Protoc 8, 325–344 (2013). https://doi.org/10.1038/nprot.2012.155
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2012.155
This article is cited by
-
Study of the asymmetric organocatalyzed [3+2] annulation of cyclopropenone and β-keto ester
Chemistry of Heterocyclic Compounds (2018)
-
3,5-Dinitrobenzoyl-9-amino-9-deoxy-9-epiquinine as Pirkle-Anion Exchange Hybrid-Type Chiral Selector in High-Performance Liquid Chromatography
Chromatographia (2017)
-
Cinchona-Alkaloids Based Isoselenazolones: Synthesis and Their Catalytic Reactivity in Asymmetric Bromolactonization of Alkenoic Acid
Proceedings of the National Academy of Sciences, India Section A: Physical Sciences (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.