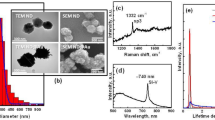
Abstract
Label and label-free methods to image carbon-based nanomaterials exist. However, label-based approaches are limited by the risk of tag detachment over time, and label-free spectroscopic methods have slow imaging speeds, weak photoluminescence signals and strong backgrounds. Here, we present a label-free mass spectrometry imaging method to detect carbon nanotubes, graphene oxide and carbon nanodots in mice. The large molecular weights of nanoparticles are difficult to detect using conventional mass spectrometers, but our method overcomes this problem by using the intrinsic carbon cluster fingerprint signal of the nanomaterials. We mapped and quantified the sub-organ distribution of the nanomaterials in mice. Our results showed that most carbon nanotubes and nanodots were found in the outer parenchyma of the kidney, and all three materials were seen in the red pulp of the spleen. The highest concentrations of nanotubes in the spleen were found within the marginal zone.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Jariwala, D., Sangwan, V. K., Lauhon, L. J., Marks, T. J. & Hersam, M. C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 42, 2824–2860 (2013).
Allen, M. J., Tung, V. C. & Kaner, R. B. Honeycomb carbon: a review of graphene. Chem. Rev. 110, 132–145 (2010).
Miyako, E. et al. Carbon nanotube–liposome supramolecular nanotrains for intelligent molecular-transport systems. Nature Commun. 3, 1226 (2012).
Cai, D. et al. Highly efficient molecular delivery into mammalian cells using carbon nanotube spearing. Nature Methods 2, 449–454 (2005).
Baker, S. N. & Baker, G. A. Luminescent carbon nanodots: emergent nanolights. Angew. Chem. Int. Ed. 49, 6726–6744 (2010).
Abarrategi, A. et al. Multiwall carbon nanotube scaffolds for tissue engineering purposes. Biomaterials 29, 94–102 (2008).
Yang, X., Ren, J., Qu, K. & Qu, X. Using graphene oxide high near-infrared absorbance for photothermal treatment of Alzheimer's disease. Adv. Mater. 24, 1722–1728 (2012).
Srikanth, M. & Kessler, J. A. Nanotechnology-novel therapeutics for CNS disorders. Nature Rev. Neurol. 8, 307–318 (2012).
Kostarelos, K., Bianco, A. & Prato, M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nature Nanotech. 4, 626–633 (2009).
Hu, X. & Zhou, Q. Health and ecosystem risks of graphene. Chem. Rev. 113, 3815–3835 (2013).
Mao, H. Y. et al. Graphene: promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 113, 3407–3424 (2013).
Schipper, M. L. et al. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nature Nanotech. 3, 216–221 (2008).
Georgin, D. et al. Preparation of 14C-labeled multiwalled carbon nanotubes for biodistribution investigations. J. Am. Chem. Soc. 131, 14658–14659 (2009).
Yang, S. et al. Biodistribution of pristine single-walled carbon nanotubes in vivo. J. Phys. Chem. C 111, 17761–17764 (2007).
Singh, R. et al. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc. Natl Acad. Sci. USA 103, 3357–3362 (2006).
Huang, X. L. et al. Effect of injection routes on the biodistribution, clearance, and tumor uptake of carbon dots. ACS Nano 7, 5684–5693 (2013).
Liu, Z. et al. Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy. Proc. Natl Acad. Sci. USA 105, 1410–1415 (2008).
Leeuw, T. K. et al. Single-walled carbon nanotubes in the intact organism—near-IR imaging and biocompatibility studies in Drosophila. Nano Lett. 7, 2650–2654 (2007).
Welsher, K., Sherlock, S. P. & Dai, H. Deep-tissue anatomical imaging of mice using carbon nanotube fluorophores in the second near-infrared window. Proc. Natl Acad. Sci. USA 108, 8943–8948 (2011).
Avti, P. K. et al. Detection, mapping, and quantification of single walled carbon nanotubes in histological specimens with photoacoustic microscopy. PLoS ONE 7, e35064 (2012).
Zhang, H. F., Maslov, K., Stoica, G. & Wang, L. V. Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging. Nature Biotechnol. 24, 848–851 (2006).
Tong, L. et al. Label-free imaging of semiconducting and metallic carbon nanotubes in cells and mice using transient absorption microscopy. Nature Nanotech. 7, 56–61 (2012).
Berry, K. A. Z. et al. MALDI imaging of lipid biochemistry in tissues by mass spectrometry. Chem. Rev. 111, 6491–6512 (2011).
Cornett, D. S., Reyzer, M. L., Chaurand, P. & Caprioli, R. M. MALDI imaging mass spectrometry: molecular snapshots of biochemical systems. Nature Methods 4, 828–833 (2007).
Chughtai, K. & Heeren, R. M. A. Mass spectrometric imaging for biomedical tissue analysis. Chem. Rev. 110, 3237–3277 (2010).
Ellis, S. R., Bruinen, A. L. & Heeren, R. M. A critical evaluation of the current state-of-the-art in quantitative imaging mass spectrometry. Anal. Bioanal. Chem. 406, 1275–1289 (2014).
Yan, B. et al. Multiplexed imaging of nanoparticles in tissues using laser desorption/ionization mass spectrometry. J. Am. Chem. Soc. 135, 12564–12567 (2013).
Zhu, Z-J., Ghosh, P. S., Miranda, O. R., Vachet, R. W. & Rotello, V. M. Multiplexed screening of cellular uptake of gold nanoparticles using laser desorption/ionization mass spectrometry. J. Am. Chem. Soc. 130, 14139–14143 (2008).
Orden, A. V. & Saykally, R. J. Small carbon clusters: spectroscopy, structure, and energetics. Chem. Rev. 98, 2313–2357 (1998).
Belau, L. et al. Ionization thresholds of small carbon clusters: tunable VUV experiments and theory. J. Am. Chem. Soc. 129, 10229–10243 (2007).
Chen, S. et al. Carbon nanodots as a matrix for the analysis of low-molecular-weight molecules in both positive- and negative-ion matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and quantification of glucose and uric acid in real samples. Anal. Chem. 85, 6646–6652 (2013).
Liu, J. H., Yang, S. T., Wang, H. F. & Liu, Y. F. Advances in biodistribution study and tracing methodology of carbon nanotubes. J. Nanosci. Nanotechnol. 10, 8469–8481 (2010).
Zhang, X. et al. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon 49, 986–995 (2011).
Tao, H. et al. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small 8, 281–290 (2011).
Lacerda, L. et al. Tissue histology and physiology following intravenous administration of different types of functionalized multiwalled carbon nanotubes. Nanomedicine 3, 149–161 (2008).
Choi, H. S. et al. Renal clearance of quantum dots. Nature Biotechnol. 25, 1165–1170 (2007).
Longmire, M., Choyke, P. L. & Kobayashi, H. Clearance properties of nano-sized particles and molecules as imaging agents: considerations and caveats. Nanomedicine 3, 703–717 (2008).
Zhou, C. et al. Near-infrared emitting radioactive gold nanoparticles with molecular pharmacokinetics. Angew. Chem. Int. Ed. 51, 10118–10122 (2012).
Mebius, R. E. & Kraal, G. Structure and function of the spleen. Nature Rev. Immunol. 5, 606–616 (2005).
Szájli, E., Fehér, T. & Medzihradszky, K. F. Investigating the quantitative nature of MALDI-TOF MS. Mol. Cell. Proteomics 7, 2410–2418 (2008).
Duncan, M. W., Roder, H. & Hunsucker, S. W. Quantitative matrix-assisted laser desorption/ionization mass spectrometry. Brief. Funct. Genomic Proteomic 7, 355–370 (2008).
Pirman, D. A., Reich, R. F., Kiss, A., Heeren, R. M. & Yost, R. A. Quantitative MALDI tandem mass spectrometric imaging of cocaine from brain tissue with a deuterated internal standard. Anal. Chem. 85, 1081–1089 (2013).
Liu, J-H. et al. Effect of size and dose on the biodistribution of graphene oxide in mice. Nanomedicine 7, 1801–1812 (2012).
Almeida, J. P. M., Chen, A. L., Foster, A. & Drezek, R. In vivo biodistribution of nanoparticles. Nanomedicine 6, 815–835 (2011).
Koeniger, S. L. et al. A quantitation method for mass spectrometry imaging. Rapid Commun. Mass Spectrom. 25, 503–510 (2011).
Lagarrigue, M. et al. Localization and in situ absolute quantification of chlordecone in the mouse liver by MALDI imaging. Anal. Chem. 86, 5775–5783 (2014).
Edelson-Averbukh, M., Pipkorn, R. & Lehmann, W. D. Phosphate group-driven fragmentation of multiply charged phosphopeptide anions. Improved recognition of peptides phosphorylated at serine, threonine, or tyrosine by negative ion electrospray tandem mass spectrometry. Anal. Chem. 78, 1249–1256 (2006).
Kauppila, T. J., Kotiaho, T., Kostiainen, R. & Bruins, A. P. Negative ion-atmospheric pressure photoionization-mass spectrometry. J. Am. Soc. Mass Spectrom. 15, 203–211 (2004).
National Research Council Guide for the Care and Use of Laboratory Animals: Eighth Edition (The National Academies Press, 2011).
Acknowledgements
This work was supported by grants from the National Natural Sciences Foundation of China (grants 21127901, 21321003, 21175139, 21305144 and 21205123) and the Chinese Academy of Sciences. A.B-T. acknowledges support from ‘The Ohio State University Start-up Funds’. The authors thank R. Graham Cooks and A. Tao for discussions.
Author information
Authors and Affiliations
Contributions
Z.X.N. and S.M.C. conceived and designed the experiments. S.M.C. performed the experiments. C.Q.X., H.H.L. and Q.H. helped with animal care. S.M.C. and Q.Q.W. analysed the data. Q.Q.W. and H.H.L. contributed to cell culture. J.H. helped with TEM analysis. S.M.C., A.B-T. and Z.X.N. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary Information (PDF 2590 kb)
Rights and permissions
About this article
Cite this article
Chen, S., Xiong, C., Liu, H. et al. Mass spectrometry imaging reveals the sub-organ distribution of carbon nanomaterials. Nature Nanotech 10, 176–182 (2015). https://doi.org/10.1038/nnano.2014.282
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2014.282
This article is cited by
-
Effect of sp3/sp2 carbon ratio and hydrodynamic size on the biodistribution kinetics of nanodiamonds in mice via intravenous injection
Particle and Fibre Toxicology (2023)
-
Microlensed fiber allows subcellular imaging by laser-based mass spectrometry
Nature Protocols (2023)
-
Nanomedicine and graphene-based materials: advanced technologies for potential treatments of diseases in the developing nervous system
Pediatric Research (2022)
-
Respiratory and systemic impacts following MWCNT inhalation in B6C3F1/N mice
Particle and Fibre Toxicology (2021)
-
Novel Green Silver Nanoparticles as Matrix in the Detection of Small Molecules Using Matrix-Assisted Laser Desorption Ionization Mass Spectrometry (MALDI-MS)
Journal of Pharmaceutical Innovation (2021)