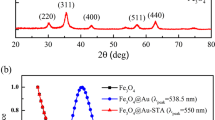
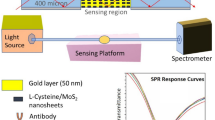
Abstract
Nanoparticles containing magnetic materials, such as magnetite (Fe3O4), are particularly useful for imaging and separation techniques. As these nanoparticles are generally considered to be biologically and chemically inert, they are typically coated with metal catalysts, antibodies or enzymes to increase their functionality as separation agents. Here, we report that magnetite nanoparticles in fact possess an intrinsic enzyme mimetic activity similar to that found in natural peroxidases, which are widely used to oxidize organic substrates in the treatment of wastewater or as detection tools. Based on this finding, we have developed a novel immunoassay in which antibody-modified magnetite nanoparticles provide three functions: capture, separation and detection. The stability, ease of production and versatility of these nanoparticles makes them a powerful tool for a wide range of potential applications in medicine, biotechnology and environmental chemistry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Bergemann, C. et al. Magnetic ion-exchange nano- and microparticles for medical, biochemical and molecular biological applications. Magn. Magn. Mater. 194, 45–52 (1999).
Safarik, I. et al. Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn. Res. Technol. 2, 7 (2004).
Morishita, N. et al. MNPs with surface modification enhanced gene delivery of HVJ-E vector. Biochem. Biophys. Res. Commun. 334, 1121–1126 (2005).
Ito, A. et al. Construction and delivery of tissue-engineered human retinal pigment epithelial cell sheets using magnetite nanoparticles and magnetic force. Tissue Eng. 11, 489–496 (2005).
Brahler, M. et al. Magnetite-loaded carrier erythrocytes as contrast agents for magnetic resonance imaging. Nano Lett. 6, 2505–2509 (2006).
de Vries, I. J. et al. Magnetic resonance tracking of dendritic cells in melanoma patients for monitoring of cellular therapy. Nature Biotechnol. 23, 1407–1413 (2005).
Denis, M. C. et al. Imaging inflammation of the pancreatic islets in type 1 diabetes. Proc. Natl Acad. Sci. USA 101, 12634–12639 (2004).
Chemla, Y. R. et al. Ultrasensitive magnetic biosensor for homogeneous immunoassay. Proc. Natl Acad. Sci. USA 97, 14268–14272 (2000).
Hirsch, L. R. et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl Acad. Sci. USA 100, 13549–13554 (2003).
Yoon, T. J. et al. MNPs as a catalyst vehicle for simple and easy recycling. New J. Chem. 27, 227–229 (2003).
Stevens, P. D. et al. Superparamagnetic nanoparticle-supported catalysis of Suzuki cross-coupling reactions. Org. Lett. 7, 2085–2088 (2005).
Hu, A. et al. Magnetically recoverable chiral catalysts immobilized on magnetite nanoparticles for asymmetric hydrogenation of aromatic ketones. J. Am. Chem. Soc. 127, 12486–12487 (2005).
Tsang, S. C. et al. Magnetically separable, carbon-supported nanocatalysts for the manufacture of fine chemicals. Angew. Chem. Int. Edn 43, 5645–5649 (2004).
Yang, H. H. et al. Magnetite-containing spherical silica nanoparticles for biocatalysis and bioseparations. Anal. Chem. 76, 1316–1321 (2004).
Jun, C. H. et al. Demonstration of a magnetic and catalytic Co@Pt nanoparticle as a dual-function nanoplatform. Chem. Commun. 1619–1621 (2006).
Henriksen, A. et al. The structures of the horseradish peroxidase C-ferulic acid complex and the ternary complex with cyanide suggest how peroxidases oxidize small phenolic substrates. J. Biol. Chem. 274, 35005–35011 (1999).
Kvaratskhelia, M. et al. Purification and characterization of a novel class III peroxidase isoenzyme from tea leaves. Plant. Physiol. 114, 1237–1245 (1997).
Kariya, K. et al. Purification and some properties of peroxidases of rat bone marrow. Biochim. Biophys. Acta. 911, 95–101 (1987).
Mathy-Hartert, M. et al. Purification of myeloperoxidase from equine polymorphonuclear leucocytes. Can. J. Vet. Res. 62, 127–132 (1998).
Hsueh, C. L., Huang, Y. H., Wang, C. C. & Chen, C. Y. Degradation of azo dyes using low iron concentration of Fenton and Fenton-like system. Chemosphere 58,1409–1414 (2005).
Yamada, H. et al. Proton balance in conversions between five oxidation–reduction states of horseradish peroxidases. Arch. Biochem. Biophys. 165, 728–738 (1974).
Porter, D. J. T. & Bright J. H. The mechanism of oxidation of nitroalkanes by horseradish peroxidase. J. Biol. Chem. 258, 9913–9924 (1982).
Bruce, I. J. et al. Synthesis, characterisation and application of silica–magnetite nanocomposites. J. Magn. Magn. Mater. 284, 145–160 (2004).
Yang, Y. D. et al. Influence of preparation parameters and characterization of nano-compound superparamagnetic iron oxide particle in dextran system. J. Inorg. Mater. 20, 226–229 (2005).
Sun, Y. K. et al. An improved way to prepare superparamagnetic magnetite–silica core–shell nanoparticles for possible biological application. J. Magn. Magn. Mater. 285, 65–70 (2005).
Zhu, Q. Z. et al. Fluorescence immunoassay of alpha-1-fetoprotein with hemin as a mimetic enzyme labeling reagent. Anal. Chem. 362, 537–540 (1998).
Zhang, G. F. et al. Hematin as a peroxidase substitute in hydrogen peroxide determinations. Anal. Chem. 64, 517–522 (1992).
Bonar-Law, R. P. et al. Polyol recognition by a steroid-capped porphyrin enhancement and modulation of misfitguest binding by added water or methanol. J. Am. Chem. Soc. 117, 259–271 (1995).
Wang, Q. L. et al. Highly sensitive fluorescent enhance assay for ascorbic acid. Anal. Chem. 28, 1229–1232 (2000).
Liu, Z. H. et al. Highly sensitive spectrofluorimetric determination of hydrogen peroxide with β-cyclodextrin–hemin as catalyst. Analyst 124, 173–176 (1999).
Ci, Y. X. et al. Chemiluminescence investigation of the interaction of metalloporphyrins with nucleic acids. Anal. Chim. Acta. 282, 695–701 (1993).
Tie, J. K. et al. Application of enzyme-field effect transistor sensor arrays as detectors in a flow-injection analysis system for simultaneous monitoring of medium components. Part II. Monitoring of cultivation processes. Anal. Chim. Acta. 300, 25–31 (1995).
Cai, Y. X. et al. Fluorimetric mimetic enzyme immunoassay of methotrexate Fresenius. Anal. Chem. 349, 317–319 (1994).
Zhu, C. Q. et al. Application of manganese–teasulfonatophthalocyanine as a new mimetic peroxidase in the determination of hydrogen peroxide in marine surface water samples with p-hydroxyphenglacetic acid as a substrate. Anal. Sci. 16, 253–256 (2000).
Cheng, J. et al. Horseradish peroxidase immobilized on aluminium-pillared inter-layered clay for the catalytic oxidation of phenolic wastewater. Water Res. 40, 283–290 (2006).
Ren, C. et al. Hydrogen peroxide sensor based on horseradish peroxidase immobilized on a silver nanoparticles/cysteamine/gold electrode. Anal. Bioanal. Chem. 381, 1179–1185 (2005).
Deng, H. et al. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Edn 44, 2782–2785 (2005).
Ma, M. et al. Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J. Magn. Magn. Mater. 268, 33–39 (2004).
Acknowledgements
We thank Sishen Xie and Chen Wang for helpful discussions. This work is partly supported by grants from the National Natural Science Foundation of China (NSFC) (90406020, 30670428), the Knowledge Innovation Program of the Chinese Academy of Sciences (kjcx-yw-m02, kjcx2-sw-h12), the Chinese Ministry of Sciences 973 Project (2006CB933204, 2006CB500703, 2006CB910901, 2006CB910903), and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
Author information
Authors and Affiliations
Contributions
L. G., J. Zhuang, S. P. and X. Y. designed the research. L. G., J. Zhuang, J. F., D. Y. and J. Zhang performed the research. L. N., Y. Z., N. G., and T. W. contributed new reagents and analytic tools. L. G., J. Zhuang, J. Zhang and X. Y. analysed the data. L. G., S. P. and X. Y. wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figure S1 (PDF 115 kb)
Rights and permissions
About this article
Cite this article
Gao, L., Zhuang, J., Nie, L. et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotech 2, 577–583 (2007). https://doi.org/10.1038/nnano.2007.260
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nnano.2007.260
This article is cited by
-
A natural biogenic nanozyme for scavenging superoxide radicals
Nature Communications (2024)
-
Protective effects of Pt-N-C single-atom nanozymes against myocardial ischemia-reperfusion injury
Nature Communications (2024)
-
A highly sensitive colorimetric DNA sensor for MicroRNA-155 detection: leveraging the peroxidase-like activity of copper nanoparticles in a double amplification strategy
Microchimica Acta (2024)
-
Hydrogen iron oxide from an Acinetobacter strain exhibiting intrinsic peroxidase-like activity and its catalytic mechanism and applications
Biomass Conversion and Biorefinery (2024)
-
Poly-β-cyclodextrin strengthen Pr6O11 porous oxidase mimic for dual-channel visual recognition of bioactive cysteine and Fe2+
Analytical and Bioanalytical Chemistry (2024)