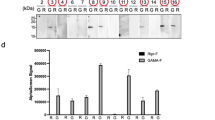
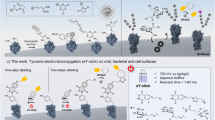
Abstract
We report a highly specific, robust and rapid new method for labeling cell surface proteins with biophysical probes. The method uses the Escherichia coli enzyme biotin ligase (BirA), which sequence-specifically ligates biotin to a 15-amino-acid acceptor peptide (AP). We report that BirA also accepts a ketone isostere of biotin as a cofactor, ligating this probe to the AP with similar kinetics and retaining the high substrate specificity of the native reaction. Because ketones are absent from native cell surfaces, AP-fused recombinant cell surface proteins can be tagged with the ketone probe and then specifically conjugated to hydrazide- or hydroxylamine-functionalized molecules. We demonstrate this two-stage protein labeling methodology on purified protein, in the context of mammalian cell lysate, and on epidermal growth factor receptor (EGFR) expressed on the surface of live HeLa cells. Both fluorescein and a benzophenone photoaffinity probe are incorporated, with total labeling times as short as 20 min.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Keppler, A., Pick, H., Arrivoli, C., Vogel, H. & Johnsson, K. Labeling of fusion proteins with synthetic fluorophores in live cells. Proc. Natl. Acad. Sci. USA 101, 9955–9959 (2004).
Miller, L.W., Sable, J., Goelet, P., Sheetz, M.P. & Cornish, V.W. Methotrexate conjugates: a molecular in vivo protein tag. Angew. Chem. Int. Edn. Engl. 43, 1672–1675 (2004).
Marks, K.M., Braun, P.D. & Nolan, G.P. A general approach for chemical labeling and rapid, spatially controlled protein inactivation. Proc. Natl. Acad. Sci. USA 101, 9982–9987 (2004).
George, N., Pick, H., Vogel, H., Johnsson, N. & Johnsson, K. Specific labeling of cell surface proteins with chemically diverse compounds. J. Am. Chem. Soc. 126, 8896–8897 (2004).
Yin, J., Liu, F., Li, X. & Walsh, C.T. Labeling proteins with small molecules by site-specific posttranslational modification. J. Am. Chem. Soc. 126, 7754–7755 (2004).
Adams, S.R. et al. New biarsenical ligands and tetracysteine motifs for protein labeling in vitro and in vivo: Synthesis and biological applications. J. Am. Chem. Soc. 124, 6063–6076 (2002).
Marks, K.M., Rosinov, M. & Nolan, G.P. In vivo targeting of organic calcium sensors via genetically selected peptides. Chem. Biol. 11, 347–356 (2004).
Guignet, E.G., Hovius, R. & Vogel, H. Reversible site-selective labeling of membrane proteins in live cells. Nat. Biotechnol. 22, 440–444 (2004).
Chen, I. & Ting, A.Y. Site-specific labeling of proteins with small molecules in live cells. Curr. Opin. Biotech. (in the press).
Beckett, D., Kovaleva, E. & Schatz, P.J. A minimal peptide substrate in biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci. 8, 921–929 (1999).
de Boer, E. et al. Efficient biotinylation and single-step purification of tagged transcription factors in mammalian cells and transgenic mice. Proc. Natl. Acad. Sci. USA 100, 7480–7485 (2003).
Mahal, L.K., Yarema, K.J. & Bertozzi, C.R. Engineering chemical reactivity on cell surfaces through oligosaccharide biosynthesis. Science 276, 1125–1128 (1997).
Baraldi, P.G. et al. Synthesis of sulfur-containing carbaprostacyclin analogs. Gazz. Chim. Ital. 114, 177–183 (1984).
Lavielle, S., Bory, S., Moreau, B., Luche, M.J. & Marquet, A. Total synthesis of biotin based on stereoselective alkylation of sulfoxides. J. Am. Chem. Soc. 100, 1558–1563 (1978).
Chapman-Smith, A., Morris, T.W., Wallace, J.C. & Cronan, J.E., Jr. Molecular recognition in a post-translational modification of exceptional specificity. Mutants of the biotinylated domain of acetyl-CoA carboxylase defective in recognition by biotin protein ligase. J. Biol. Chem. 274, 1449–1457 (1999).
Nauman, D.A. & Bertozzi, C.R. Kinetic parameters for small-molecule drug delivery by covalent cell surface targeting. Biochim. Biophys. Acta 1568, 147–154 (2001).
Lax, I. et al. Epidermal growth factor (EGF) induces oligomerization of soluble, extracellular, ligand-binding domain of EGF receptor. A low resolution projection structure of the ligand-binding domain. J. Biol. Chem. 266, 13828–13833 (1991).
Brock, R., Hamelers, I.H. & Jovin, T.M. Comparison of fixation protocols for adherent cultured cells applied to a GFP fusion protein of the epidermal growth factor receptor. Cytometry 35, 353–362 (1999).
Reynolds, A.R., Tischer, C., Verveer, P.J., Rocks, O. & Bastiaens, P.I. EGFR activation coupled to inhibition of tyrosine phosphatases causes lateral signal propagation. Nat. Cell Biol. 5, 447–453 (2003).
Zhang, Z. et al. A new strategy for the site-specific modification of proteins in vivo. Biochemistry 42, 6735–6746 (2003).
Huff, T. et al. Thymosin β(4) serves as a glutaminyl substrate of transglutaminase. Labeling with fluorescent dansylcadaverine does not abolish interaction with G-actin. FEBS Lett 464, 14–20 (1999).
Dutton, A. & Singer, S.J. Crosslinking and labeling of membrane proteins by transglutaminase-catalyzed reactions. Proc. Natl. Acad. Sci. USA 72, 2568–2571 (1975).
Mao, H., Hart, S.A., Schink, A. & Pollok, B.A. Sortase-mediated protein ligation: a new method for protein engineering. J. Am. Chem. Soc. 126, 2670–2671 (2004).
Acknowledgements
Funding was provided by the US National Institutes of Health (K22-HG002671-01), EJLB Foundation and Massachusetts Institute of Technology. I.C. was supported by a National Science Foundation predoctoral fellowship and a Wyeth Pharmaceuticals fellowship, and M.H. was supported by an MIT-Merck postdoctoral fellowship. We thank Tanabe USA for biotin, D. Beckett for the BirA plasmid and helpful advice, K.D. Wittrup for the EGFR plasmid, J.M. Baskin, E. McNeill and K.H. Riesenburger for assistance, and J.E. Wilson and G.C. Fu for use of their chiral HPLC. We also thank S.S. Licht and R.Y. Tsien for helpful advice.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Massachusetts Institute of Technology is seeking to file a patent application covering part of the information contained in the paper.
Supplementary information
Supplementary Fig. 1
Comparison of our labeling method (ketone 1 followed by FH) to antibody detection (anti-pentahistidine mouse monoclonal followed by fluorescein-conjugated secondary antibody) for visualization of CFP-AP in lysate. (PDF 88 kb)
Supplementary Fig. 2
Analysis of the effect of the AP tag on EGFR distribution and function. (PDF 4787 kb)
Rights and permissions
About this article
Cite this article
Chen, I., Howarth, M., Lin, W. et al. Site-specific labeling of cell surface proteins with biophysical probes using biotin ligase. Nat Methods 2, 99–104 (2005). https://doi.org/10.1038/nmeth735
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmeth735
This article is cited by
-
Designer installation of a substrate recruitment domain to tailor enzyme specificity
Nature Chemical Biology (2023)
-
Molecular mechanism of phosphopeptide neoantigen immunogenicity
Nature Communications (2023)
-
Ligand-directed two-step labeling to quantify neuronal glutamate receptor trafficking
Nature Communications (2021)
-
Live cell PNA labelling enables erasable fluorescence imaging of membrane proteins
Nature Chemistry (2021)
-
Quantifying residue-specific conformational dynamics of a highly reactive 29-mer peptide
Scientific Reports (2020)