Abstract
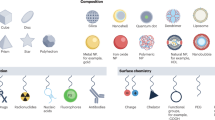
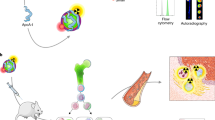
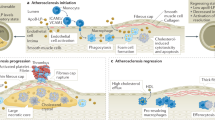
Nanomaterials have much to offer, not only in deciphering innate immune cell biology and tracking cells, but also in advancing personalized clinical care by providing diagnostic and prognostic information, quantifying treatment efficacy and designing better therapeutics. This Review presents different types of nanomaterial, their biological properties and their applications for imaging macrophages in human diseases, including cancer, atherosclerosis, myocardial infarction, aortic aneurysm, diabetes and other conditions. We anticipate that future needs will include the development of nanomaterials that are specific for immune cell subsets and can be used as imaging surrogates for nanotherapeutics. New in vivo imaging clinical tools for noninvasive macrophage quantification are thus ultimately expected to become relevant to predicting patients' clinical outcome, defining treatment options and monitoring responses to therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Shi, C. et al. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 34, 590–601 (2011).
Swirski, F. K. et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 (2009).
Cortez-Retamozo, V. et al. Origins of tumor-associated macrophages and neutrophils. Proc. Natl Acad. Sci. USA 109, 2491–2496 (2012).
Dutta, P. et al. Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 (2012).
Jenkins, S. J. et al. Local macrophage proliferation, rather than recruitment from the blood, is a signature of Th2 inflammation. Science 332, 1284–1288 (2011).
Cortez-Retamozo, V. et al. Angiotensin II drives the production of tumor-promoting macrophages. Immunity 38, 296–308 (2013).
Qian, B. Z. et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature 475, 222–225 (2011).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Weissleder, R. & Pittet, M. J. Imaging in the era of molecular oncology. Nature 452, 580–589 (2008).
Qian, B. Z. & Pollard, J. W. Macrophage diversity enhances tumor progression and metastasis. Cell 141, 39–51 (2010).
Moore, K. J. & Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 (2011).
Ji, H. et al. Arthritis critically dependent on innate immune system players. Immunity 16, 157–168 (2002).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Bhargava, P. & Lee, C. H. Role and function of macrophages in the metabolic syndrome. Biochem. J. 442, 253–262 (2012).
Cheng, Z., Al Zaki, A., Hui, J. Z., Muzykantov, V. R. & Tsourkas, A. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science 338, 903–910 (2012).
Li, C. A targeted approach to cancer imaging and therapy. Nature. Mater. 13, 110–115 (2014).
Shao, H., Yoon, T. J., Liong, M., Weissleder, R. & Lee, H. Magnetic nanoparticles for biomedical NMR-based diagnostics. Beilstein J. Nanotechnol. 1, 142–154 (2010).
Colombo, M. et al. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 41, 4306–4334 (2012).
Jacobs, I. S. Magnetic materials and applications: a quarter-century overview. J. Appl. Phys. 50, 7294–7307 (1979).
Shen, T., Weissleder, R., Papisov, M., Bogdanov, A. J. & Brady, T. J. Monocrystalline iron oxide nanocompounds (MION): physicochemical properties. Magn. Reson. Med. 29, 599–604 (1993).
Chourpa, I., Douziech-Eyrolles, L. & Ngaboni-Okassa, L. Molecular composition of iron oxide nanoparticles, precursors for magnetic drug targeting, as characterized by confocal Raman microspectroscopy. Analyst 130, 1395–1403 (2005).
Yoon, T. J., Lee, H., Shao, H. & Weissleder, R. Highly magnetic core–shell nanoparticles with a unique magnetization mechanism. Angew. Chem. Int. Ed. 50, 4663–4666 (2011).
Gallo, J., Genicio, N. & Penades, S. Uptake and intracellular fate of fluorescent-magnetic glyco-nanoparticles. Adv. Healthc. Mater. 1, 302–307 (2012).
Yu, H. et al. Dumbbell-like bifunctional Au–Fe3O4 nanoparticles. Nano. Lett. 5, 379–382 (2005).
Zeng, Q., Baker, I., Loudis, J. A., Liao, Y. & Hoopes, P. J. Fe/Fe oxide nanocomposite particles with large specific absorption rate for hyperthermia. Appl. Phys. Lett. 90, 233112 (2007).
Yoon, T. J., Lee, H., Shao, H., Hilderbrand, S. A. & Weissleder, R. Multicore assemblies potentiate magnetic properties of biomagnetic nanoparticles. Adv. Mater. 23, 4793–4797 (2011).
Jordan, A., Scholz, R., Wust, P. & Fähling, H. Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 201, 413–419 (1999).
Gupta, A. K. & Gupta, M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 26, 3995–4021 (2005).
Wahajuddin, Arora, S. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 7, 3445–3471 (2012).
Jedlovszky-Hajdu, A., Bombelli, F. B., Monopoli, M. P., Tombacz, E. & Dawson, K. A. Surface coatings shape the protein corona of SPIONs with relevance to their application in vivo. Langmuir 28, 14983–14991 (2012).
Harisinghani, M., Ross, R. W., Guimaraes, A. R. & Weissleder, R. Utility of a new bolus-injectable nanoparticle for clinical cancer staging. Neoplasia 9, 1160–1165 (2007).
Tassa, C., Shaw, S. Y. & Weissleder, R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc. Chem. Res. 44, 842–852 (2011).
Taupitz, M. et al. New generation of monomer-stabilized very small superparamagnetic iron oxide particles (VSOP) as contrast medium for MR angiography: preclinical results in rats and rabbits. J. Magn. Reson. Imaging 12, 905–911 (2000).
Sheashaa, H. et al. Parenteral iron therapy in treatment of anemia in end-stage renal disease patients: a comparative study between iron saccharate and gluconate. Nephron. Clin. Pract. 99, c97–c101 (2005).
Wei, H. et al. Compact zwitterion-coated iron oxide nanoparticles for biological applications. Nano. Lett. 12, 22–25 (2012).
Benbenishty-Shamir, H., Gilert, R., Gotman, I., Gutmanas, E. Y. & Sukenik, C. N. Phosphonate-anchored monolayers for antibody binding to magnetic nanoparticles. Langmuir 27, 12082–12089 (2011).
Portet, D., Denizot, B., Rump, E., Lejeune, J. J. & Jallet, P. Nonpolymeric coatings of iron oxide colloids for biological use as magnetic resonance imaging contrast agents. J. Colloid Interface Sci. 238, 37–42 (2001).
Skajaa, T. et al. The biological properties of iron oxide core high-density lipoprotein in experimental atherosclerosis. Biomaterials 32, 206–213 (2011).
Frias, J. C., Ma, Y., Williams, K. J., Fayad, Z. A. & Fisher, E. A. Properties of a versatile nanoparticle platform contrast agent to image and characterize atherosclerotic plaques by magnetic resonance imaging. Nano. Lett. 6, 2220–2224 (2006).
Ingram, D. R. et al. Superparamagnetic nanoclusters coated with oleic acid bilayers for stabilization of emulsions of water and oil at low concentration. J. Colloid Interface Sci. 351, 225–232 (2010).
Weissleder, R., Kelly, K., Sun, E. Y., Shtatland, T. & Josephson, L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nature Biotechnol. 23, 1418–1423 (2005).
Akinc, A. et al. A combinatorial library of lipid-like materials for delivery of RNAi therapeutics. Nature Biotechnol. 26, 561–569 (2008).
Leuschner, F. et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nature Biotechnol. 29, 1005–1010 (2011).
Ghosh, D. et al. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nature Nanotech. 7, 677–682 (2012).
Maillard, N., Clouet, A., Darbre, T. & Reymond, J. L. Combinatorial libraries of peptide dendrimers: design, synthesis, on-bead high-throughput screening, bead decoding and characterization. Nature Protoc. 4, 132–142 (2009).
Tassa, C. et al. Binding affinity and kinetic analysis of targeted small molecule-modified nanoparticles. Bioconjug. Chem. 21, 14–19 (2010).
Epa, V. C. et al. Modeling biological activities of nanoparticles. Nano. Lett. 12, 5808–5812 (2012).
Fourches, D. et al. Quantitative nanostructure-activity relationship modeling. ACS Nano 4, 5703–5712 (2010).
Korosoglou, G. et al. Off-resonance angiography: a new method to depict vessels—phantom and rabbit studies. Radiology 249, 501–509 (2008).
Balducci, A. et al. A novel probe for the non-invasive detection of tumor-associated inflammation. Oncoimmunology 2, e23034 (2013).
Hitchens, T. K. et al. 19F MRI detection of acute allograft rejection with in vivo perfluorocarbon labeling of immune cells. Magn. Reson. Med. 65, 1144–1153 (2011).
Welch, M. J., Hawker, C. J. & Wooley, K. L. The advantages of nanoparticles for PET. J. Nucl. Med. 50, 1743–1746 (2009).
Keliher, E. J. et al. 89Zr-labeled dextran nanoparticles allow in vivo macrophage imaging. Bioconjug. Chem. 22, 2383–2389 (2011).
Devaraj, N. K., Keliher, E. J., Thurber, G. M., Nahrendorf, M. & Weissleder, R. 18F labeled nanoparticles for in vivo PET-CT imaging. Bioconjug. Chem. 20, 397–401 (2009).
Fukukawa, K. et al. Synthesis and characterization of core-shell star copolymers for in vivo PET imaging applications. Biomacromolecules 9, 1329–1339 (2008).
Li, A. et al. Synthesis and in vivo pharmacokinetic evaluation of degradable shell cross-linked polymer nanoparticles with poly(carboxybetaine) versus poly(ethylene glycol) surface-grafted coatings. ACS Nano 6, 8970–8982 (2012).
Liu, T. W., Macdonald, T. D., Shi, J., Wilson, B. C. & Zheng, G. Intrinsically copper-64-labeled organic nanoparticles as radiotracers. Angew. Chem. Int. Ed. 52, 13128–131231 (2012).
Bradbury, M. S. et al. Clinically-translated silica nanoparticles as dual-modality cancer-targeted probes for image-guided surgery and interventions. Integr. Biol. 5, 74–86 (2012).
Jarrett, B. R., Gustafsson, B., Kukis, D. L. & Louie, A. Y. Synthesis of 64Cu-labeled magnetic nanoparticles for multimodal imaging. Bioconjug. Chem. 19, 1496–1504 (2008).
Zeng, D. et al. 64Cu core-labeled nanoparticles with high specific activity via metal-free click chemistry. ACS Nano 6, 5209–5219 (2012).
Nahrendorf, M. et al. Detection of macrophages in aortic aneurysms by nanoparticle positron emission tomography-computed tomography. Arterioscl. Throm. Vas. 31, 750–757 (2011).
Pittet, M. J., Swirski, F. K., Reynolds, F., Josephson, L. & Weissleder, R. Labeling of immune cells for in vivo imaging using magnetofluorescent nanoparticles. Nature Protoc. 1, 73–79 (2006).
Nahrendorf, M. et al. Hybrid PET-optical imaging using targeted probes. Proc. Natl Acad. Sci. USA 107, 7910–7915 (2010).
Pittet, M. J. & Weissleder, R. Intravital imaging. Cell 147, 983–991 (2011).
Enochs, W. S. et al. MR imaging of slow axonal transport in vivo. Exp. Neurol. 123, 235–242 (1993).
Nahrendorf, M. et al. Dual channel optical tomographic imaging of leukocyte recruitment and protease activity in the healing myocardial infarct. Circ. Res. 100, 1218–1225 (2007).
De Kozak, Y. et al. Intraocular injection of tamoxifen-loaded nanoparticles: a new treatment of experimental autoimmune uveoretinitis. Eur. J. Immunol. 34, 3702–3712 (2004).
Jaulin, N., Appel, M., Passirani, C., Barratt, G. & Labarre, D. Reduction of the uptake by a macrophagic cell line of nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate). J. Drug. Target 8, 165–172 (2000).
Fuller, J. E. et al. Intracellular delivery of core-shell fluorescent silica nanoparticles. Biomaterials 29, 1526–1532 (2008).
Cong, L. et al. Uniform silica coated fluorescent nanoparticles: synthetic method, improved light stability and application to visualize lymph network tracer. PLoS One 5, e13167 (2010).
Coester, C. J., Langer, K., van Briesen, H. & Kreuter, J. Gelatin nanoparticles by two step desolvation: a new preparation method, surface modifications and cell uptake. J. Microencapsul. 17, 187–193 (2000).
Skajaa, T. et al. Quantum dot and Cy5.5 labeled nanoparticles to investigate lipoprotein biointeractions via forster resonance energy transfer. Nano. Lett. 12, 5131–5138 (2010).
Popovic, Z. et al. A nanoparticle size series for in vivo fluorescence imaging. Angew. Chem. Int. Ed. 49, 8649–8652 (2010).
Kim, S. et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nature Biotechnol. 22, 93–97 (2004).
Stroh, M. et al. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nature Med. 11, 678–682 (2005).
Hilderbrand, S. A., Shao, F., Salthouse, C., Mahmood, U. & Weissleder, R. Upconverting luminescent nanomaterials: application to in vivo bioimaging. Chem. Commun. 4188–4190 (2009).
Esipova, T. V. et al. Dendritic upconverting nanoparticles enable in vivo multiphoton microscopy with low-power continuous wave sources. Proc. Natl Acad. Sci. USA 109, 20826–20831 (2012).
Hyafil, F. et al. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nature Med. 13, 636–641 (2007).
Aillon, K. L. et al. Iodinated nanoclusters as an inhaled computed tomography contrast agent for lung visualization. Mol. Pharm. 7, 1274–1282 (2010).
Hyafil, F. et al. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. J. Nucl. Med. 50, 959–965 (2009).
Van Herck, J. L. et al. Multi-slice computed tomography with N1177 identifies ruptured atherosclerotic plaques in rabbits. Basic Res. Cardiol. 105, 51–59 (2010).
Cormode, D. P. et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology 256, 774–782 (2010).
Hutter, E. et al. Microglial response to gold nanoparticles. ACS Nano 4, 2595–2606 (2010).
Mahmoudi, M., Serpooshan, V. & Laurent, S. Engineered nanoparticles for biomolecular imaging. Nanoscale 3, 3007–3026 (2011).
Rabin, O., Manuel Perez, J., Grimm, J., Wojtkiewicz, G. & Weissleder, R. An X-ray computed tomography imaging agent based on long-circulating bismuth sulphide nanoparticles. Nature Mater. 5, 118–122 (2006).
Oh, M. H. et al. Large-scale synthesis of bioinert tantalum oxide nanoparticles for X-ray computed tomography imaging and bimodal image-guided sentinel lymph node mapping. J. Am. Chem. Soc. 133, 5508–5515 (2011).
Cormode, D. P. et al. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 8, 3715–3723 (2008).
Xia, X. R., Monteiro-Riviere, N. A. & Riviere, J. E. An index for characterization of nanomaterials in biological systems. Nature Nanotech. 5, 671–675 (2010).
Maeda, H., Nakamura, H. & Fang, J. The EPR effect for macromolecular drug delivery to solid tumors: improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo. Adv. Drug. Deliv. Rev. 65, 71–79 (2012).
Talekar, M., Kendall, J., Denny, W. & Garg, S. Targeting of nanoparticles in cancer: drug delivery and diagnostics. Anticancer Drugs 22, 949–962 (2011).
Baish, J. W. et al. Scaling rules for diffusive drug delivery in tumor and normal tissues. Proc. Natl Acad. Sci. USA 108, 1799–1803 (2011).
Chauhan, V. P., Stylianopoulos, T., Boucher, Y. & Jain, R. K. Delivery of molecular and nanoscale medicine to tumors: transport barriers and strategies. Annu. Rev. Chem. Biomol. Eng. 2, 281–298 (2011).
Stylianopoulos, T., Soteriou, K., Fukumura, D. & Jain, R. K. Cationic nanoparticles have superior transvascular flux into solid tumors: insights from a mathematical model. Ann. Biomed. Eng. 41, 68–77 (2012).
Raynal, I. et al. Macrophage endocytosis of superparamagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest. Radiol. 39, 56–63 (2004).
Araki, N., Johnson, M. T. & Swanson, J. A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell. Biol. 135, 1249–1260 (1996).
Norbury, C. C., Hewlett, L. J., Prescott, A. R., Shastri, N. & Watts, C. Class I MHC presentation of exogenous soluble antigen via macropinocytosis in bone marrow macrophages. Immunity 3, 783–791 (1995).
Lim, J. P. & Gleeson, P. A. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol. Cell Biol. 89, 836–843 (2011).
Fincham, V. J. et al. Translocation of Src kinase to the cell periphery is mediated by the actin cytoskeleton under the control of the Rho family of small G proteins. J. Cell Biol. 135, 1551–1564 (1996).
Moore, A., Weissleder, R. & Bogdanov, A. J. Uptake of dextran-coated monocrystalline iron oxides in tumor cells and macrophages. J. Magn. Reson. Imaging 7, 1140–1145 (1997).
Zimmer, C. et al. Tumor cell endocytosis imaging facilitates delineation of the glioma-brain interface. Exp. Neurol. 143, 61–69 (1997).
Leuschner, F. et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 209, 123–137 (2012).
Montet-Abou, K. et al. In vivo labelling of resting monocytes in the reticuloendothelial system with fluorescent iron oxide nanoparticles prior to injury reveals that they are mobilized to infarcted myocardium. Eur. Heart. J. 31, 1410–1420 (2010).
Gordon, S. & Martinez, F. O. Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 (2010).
Sica, A. & Mantovani, A. Macrophage plasticity and polarization: in vivo veritas. J. Clin. Invest. 122, 787–795 (2012).
Geissmann, F. et al. Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 (2010).
Nahrendorf, M. et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J. Exp. Med. 204, 3037–3047 (2007).
Carlin, L. M. et al. Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell 153, 362–375 (2013).
Cros, J. et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 33, 375–386 (2010).
Bernd, H., De Kerviler, E., Gaillard, S. & Bonnemain, B. Safety and tolerability of ultrasmall superparamagnetic iron oxide contrast agent: comprehensive analysis of a clinical development program. Invest. Radiol. 44, 336–342 (2009).
Liu, G., Gao, J., Ai, H. & Chen, X. Applications and potential toxicity of magnetic iron oxide nanoparticles. Small 9, 1533–1545 (2012).
Weissleder, R. et al. Superparamagnetic iron oxide: pharmacokinetics and toxicity. Am. J. Roentgenol. 152, 167–173 (1989).
Bourrinet, P. et al. Preclinical safety and pharmacokinetic profile of ferumoxtran-10, an ultrasmall superparamagnetic iron oxide magnetic resonance contrast agent. Invest. Radiol. 41, 313–324 (2006).
Schulze, E. et al. Cellular uptake and trafficking of a prototypical magnetic iron oxide label in vitro. Invest. Radiol. 30, 604–610 (1995).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
DeNardo, D. G. et al. Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Disc. 1, 54–67 (2011).
Steidl, C. et al. Tumor-associated macrophages and survival in classic Hodgkin's lymphoma. N. Engl. J. Med. 362, 875–885 (2010).
Mantovani, A. & Sica, A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr. Opin. Immunol. 22, 231–237 (2010).
Kircher, M. F., Mahmood, U., King, R. S., Weissleder, R. & Josephson, L. A multimodal nanoparticle for preoperative magnetic resonance imaging and intraoperative optical brain tumor delineation. Cancer Res. 63, 8122–8125 (2003).
Rainov, N. G. et al. Selective uptake of viral and monocrystalline particles delivered intra-arterially to experimental brain neoplasms. Hum. Gene Ther. 6, 1543–1552 (1995).
Zimmer, C. et al. MR imaging of phagocytosis in experimental gliomas. Radiology 197, 533–538 (1995).
Daldrup-Link, H. & Coussens, L. M. MR imaging of tumor-associated macrophages. Oncoimmunology 1, 507–509 (2012).
Daldrup-Link, H. E. et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin. Cancer Res. 17, 5695–5704 (2011).
Harisinghani, M. G. et al. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N. Engl. J. Med. 348, 2491–2499 (2003).
Harisinghani, M. G. & Weissleder, R. Sensitive, noninvasive detection of lymph node metastases. PLoS Med. 1, e66 (2004).
Mouli, S. K., Zhao, L. C., Omary, R. A. & Thaxton, C. S. Lymphotropic nanoparticle enhanced MRI for the staging of genitourinary tumors. Nature Rev. Urol. 7, 84–93 (2010).
Weissleder, R. et al. Ultrasmall superparamagnetic iron oxide: an intravenous contrast agent for assessing lymph nodes with MR imaging. Radiology 175, 494–498 (1990).
Weissleder, R. et al. MR lymphography: study of a high-efficiency lymphotrophic agent. Radiology 191, 225–230 (1994).
Wunderbaldinger, P., Josephson, L., Bremer, C., Moore, A. & Weissleder, R. Detection of lymph node metastases by contrast-enhanced MRI in an experimental model. Magn. Reson. Med. 47, 292–297 (2002).
Saokar, A. et al. Detection of lymph nodes in pelvic malignancies with computed tomography and magnetic resonance imaging. Clin. Imaging 34, 361–366 (2010).
Heesakkers, R. A. et al. Prostate cancer: detection of lymph node metastases outside the routine surgical area with ferumoxtran-10-enhanced MR imaging. Radiology 251, 408–414 (2009).
Libby, P. Inflammation in atherosclerosis. Nature 420, 868–874 (2002).
Swirski, F. K. & Nahrendorf, M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science 339, 161–166 (2013).
Winter, P. M. et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with αvβ3-integrin-targeted nanoparticles. Circulation 108, 2270–2274 (2003).
McAteer, M. A. et al. A leukocyte-mimetic magnetic resonance imaging contrast agent homes rapidly to activated endothelium and tracks with atherosclerotic lesion macrophage content. Arterioscl. Throm. Vas. 32, 1427–1435 (2012).
Nahrendorf, M. et al. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscl. Throm. Vas. 29, 1444–1451 (2009).
Lobatto, M. E., Fuster, V., Fayad, Z. A. & Mulder, W. J. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nature Rev. Drug Disc. 10, 835–852 (2011).
Tang, T. Y. et al. The ATHEROMA (atorvastatin therapy: effects on reduction of macrophage activity) study: evaluation using ultrasmall superparamagnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J. Am. Coll. Cardiol. 53, 2039–2050 (2009).
Kooi, M. E. et al. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation 107, 2453–2458 (2003).
Richards, J. M. et al. Abdominal aortic aneurysm growth predicted by uptake of ultrasmall superparamagnetic particles of iron oxide: a pilot study. Circ. Cardiovasc. Imaging 4, 274–281 (2011).
Lipinski, M. J. et al. Macrophage-specific lipid-based nanoparticles improve cardiac magnetic resonance detection and characterization of human atherosclerosis. JACC Cardiovasc. Imaging 2, 637–647 (2009).
Briley-Saebo, K. C. et al. Targeted iron oxide particles for in vivo magnetic resonance detection of atherosclerotic lesions with antibodies directed to oxidation-specific epitopes. J. Am. Coll. Cardiol. 57, 337–347 (2011).
Jaffer, F. A. et al. Cellular imaging of inflammation in atherosclerosis using magnetofluorescent nanomaterials. Mol. Imaging 5, 85–92 (2006).
Amirbekian, V. et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis noninvasively using molecular MRI. Proc. Natl Acad. Sci. USA 104, 961–966 (2007).
Majmudar, M. D. et al. Polymeric nanoparticle PET/MR imaging allows macrophage detection in atherosclerotic plaques. Circ. Res. 112, 755–761 (2013).
Weissleder, R. & Ntziachristos, V. Shedding light onto live molecular targets. Nature Med. 9, 123–128 (2003).
Buxton, D. B. Molecular imaging of aortic aneurysms. Circ. Cardiovasc. Imaging 5, 392–399 (2012).
Nahrendorf, M., Pittet, M. J. & Swirski, F. K. Monocytes: protagonists of infarct inflammation and repair after myocardial infarction. Circulation 121, 2437–2445 (2010).
Sosnovik, D. E. et al. Fluorescence tomography and magnetic resonance imaging of myocardial macrophage infiltration in infarcted myocardium in vivo. Circulation 115, 1384–1391 (2007).
Naresh, N. K. et al. Monocyte and/or macrophage infiltration of heart after myocardial infarction: MR imaging by using T1-shortening liposomes. Radiology 264, 428–435 (2012).
Flogel, U. et al. In vivo monitoring of inflammation after cardiac and cerebral ischemia by fluorine magnetic resonance imaging. Circulation 118, 140–148 (2008).
Alam, S. R. et al. Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction: early clinical experience. Circ. Cardiovasc. Imaging 5, 559–565 (2012).
Yilmaz, A. et al. Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: a human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur. Heart J. 34, 462–475 (2013).
Tsujioka, H. et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J. Am. Coll. Cardiol. 54, 130–138 (2009).
Wu, Y. L. et al. Noninvasive evaluation of cardiac allograft rejection by cellular and functional cardiac magnetic resonance. JACC Cardiovasc. Imaging 2, 731–741 (2009).
Christen, T. et al. Molecular imaging of innate immune cell function in transplant rejection. Circulation 119, 1925–1932 (2009).
Moon, H. et al. Noninvasive assessment of myocardial inflammation by cardiovascular magnetic resonance in a rat model of experimental autoimmune myocarditis. Circulation 125, 2603–2612 (2012).
Denis, M. C., Mahmood, U., Benoist, C., Mathis, D. & Weissleder, R. Imaging inflammation of the pancreatic islets in type 1 diabetes. Proc. Natl Acad. Sci. USA 101, 12634–12639 (2004).
Turvey, S. E. et al. Noninvasive imaging of pancreatic inflammation and its reversal in type 1 diabetes. J. Clin. Invest. 115, 2454–2461 (2005).
Gaglia, J. L. et al. Noninvasive imaging of pancreatic islet inflammation in type 1A diabetes patients. J. Clin. Invest. 121, 442–445 (2011).
Fu, W., Wojtkiewicz, G., Weissleder, R., Benoist, C. & Mathis, D. Early window of diabetes determinism in NOD mice, dependent on the complement receptor CRIg, identified by noninvasive imaging. Nature Immunol. 13, 361–368 (2012).
Binstadt, B. A. et al. Particularities of the vasculature can promote the organ specificity of autoimmune attack. Nature Immunol. 7, 284–292 (2006).
Dames, P. et al. Targeted delivery of magnetic aerosol droplets to the lung. Nature Nanotech. 2, 495–499 (2007).
Stoll, G. & Bendszus, M. New approaches to neuroimaging of central nervous system inflammation. Curr. Opin. Neurol. 23, 282–286 (2010).
Mueggler, T. et al. MRI signature in a novel mouse model of genetically induced adult oligodendrocyte cell death. Neuroimage 59, 1028–1036 (2012).
Tourdias, T. et al. Assessment of disease activity in multiple sclerosis phenotypes with combined gadolinium- and superparamagnetic iron oxide-enhanced MR imaging. Radiology 264, 225–233 (2012).
Hasan, D. et al. Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: a pilot study. Stroke 43, 3258–3265 (2012).
Karussis, D. et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 67, 1187–1194 (2010).
Bulte, J. W. In vivo MRI cell tracking: clinical studies. Am. J. Roentgenol. 193, 314–325 (2009).
Barnett, B. P. et al. Synthesis of magnetic resonance-, X-ray- and ultrasound-visible alginate microcapsules for immunoisolation and noninvasive imaging of cellular therapeutics. Nature Protoc. 6, 1142–1151 (2011).
Evgenov, N. V., Medarova, Z., Dai, G., Bonner-Weir, S. & Moore, A. In vivo imaging of islet transplantation. Nature Med. 12, 144–148 (2006).
Heyn, C. et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn. Reson. Med. 56, 1001–1010 (2006).
Lewin, M. et al. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nature Biotechnol. 18, 410–414 (2000).
Thu, M. S. et al. Self-assembling nanocomplexes by combining ferumoxytol, heparin and protamine for cell tracking by magnetic resonance imaging. Nature Med. 18, 463–467 (2012).
Ornberg, R. L. & Liu, H. Immunofluorescent labeling of proteins in cultured cells with quantum dot secondary antibody conjugates. Methods Mol. Biol. 374, 3–10 (2007).
Bulte, J. W. Science to practice: Can macrophage infiltration serve as a surrogate marker for stem cell viability? Radiology 264, 619–620 (2012).
Nabiev, I. et al. Nonfunctionalized nanocrystals can exploit a cell's active transport machinery delivering them to specific nuclear and cytoplasmic compartments. Nano Lett. 7, 3452–3461 (2007).
Clift, M. J., Brandenberger, C., Rothen-Rutishauser, B., Brown, D. M. & Stone, V. The uptake and intracellular fate of a series of different surface coated quantum dots in vitro. Toxicology 286, 58–68 (2011).
Awaad, A., Nakamura, M. & Ishimura, K. Imaging of size-dependent uptake and identification of novel pathways in mouse Peyer's patches using fluorescent organosilica particles. Nanomedicine 8, 627–636 (2012).
Gradishar, W. J. et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J. Clin. Oncol. 23, 7794–7803 (2005).
Kelly, K. A. et al. Unbiased discovery of in vivo imaging probes through in vitro profiling of nanoparticle libraries. Integr. Biol. 1, 311–317 (2009).
Enochs, W. S., Harsh, G., Hochberg, F. & Weissleder, R. Improved delineation of human brain tumors on MR images using a long-circulating, superparamagnetic iron oxide agent. J. Magn. Reson. Imaging 9, 228–232 (1999).
Acknowledgements
We would like to thank the National Institutes of Health for supporting this research through the National Cancer Institute Centers of Cancer Nanotechnology Excellence consortia and the National Heart Lung and Blood Institute Program of Excellence in Nanotechnology consortia. We especially would like to thank our collaborators and members of Chemical Safety Board for many helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Weissleder, R., Nahrendorf, M. & Pittet, M. Imaging macrophages with nanoparticles. Nature Mater 13, 125–138 (2014). https://doi.org/10.1038/nmat3780
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3780
This article is cited by
-
Two-dimensional nanomaterials induced nano-bio interfacial effects and biomedical applications in cancer treatment
Journal of Nanobiotechnology (2024)
-
New opportunities for RGD-engineered metal nanoparticles in cancer
Molecular Cancer (2023)
-
Bone disease imaging through the near-infrared-II window
Nature Communications (2023)
-
PET/MR imaging of inflammation in atherosclerosis
Nature Biomedical Engineering (2022)
-
Surface charge-dependent mitochondrial response to similar intracellular nanoparticle contents at sublethal dosages
Particle and Fibre Toxicology (2021)