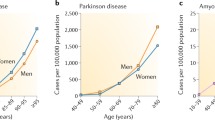
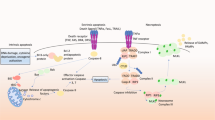
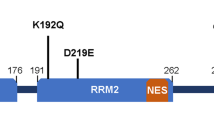
Abstract
The molecular bases underlying the pathogenesis of neurodegenerative diseases are gradually being disclosed. One problem that investigators face is distinguishing primary from secondary events. Rare, inherited mutations causing familial forms of these disorders have provided important insights into the molecular networks implicated in disease pathogenesis. Increasing evidence indicates that accumulation of aberrant or misfolded proteins, protofibril formation, ubiquitin-proteasome system dysfunction, excitotoxic insult, oxidative and nitrosative stress, mitochondrial injury, synaptic failure, altered metal homeostasis and failure of axonal and dendritic transport represent unifying events in many slowly progressive neurodegenerative disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Selkoe, D.J. Folding proteins in fatal ways. Nature 426, 900–904 (2003).
Roses, A.D. Alipoprotein E in neurology. Curr. Opin. Neurol. 4, 265–270 (1996).
Hardy, J. Amyloid, the presenilins and Alzheimer's disease. Trends Neurosci. 20, 154–159 (1997).
Selkoe, D.J. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 399, A23–A31 (1999).
Delacourte, A. & Buee, L. Tau pathology: a marker of neurodegenerative disorders. Curr. Opin. Neurol. 13, 371–376 (2000).
Rossjohn, J. et al. Crystal structure of the N-terminal, growth factor–like domain of Alzheimer amyloid precursor protein. Nat. Struct. Biol. 6, 327–331 (1999).
Barnham, K.J. et al. Structure of the Alzheimer's disease amyloid precursor binding protein. A regulator of neuronal copper homeostasis. J. Biol. Chem. 278, 17401–17407 (2003).
Watson, A.A., Fairlie, D.P. & Craik, D.J. Solution structure of methionine-oxidized amyloid β-peptide (1–40). Does oxidation affect conformational switching? Biochemistry 37, 12700–12706 (1998).
Curtain, C.C. et al. Alzheimer's disease amyloid-β binds copper and zinc to generate an allosterically ordered membrane-penetrating structure containing superoxide dismutase-like subunits. J. Biol. Chem. 276, 20466–20473 (2001).
Singleton, A., Myers, A. & Hardy, J. The law of mass action applied to neurodegenerative disease: a hypothesis concerning the etiology and pathogenesis of complex diseases. Hum. Mol. Genet. 13 (Spec. no. 1), R123–R126 (2004).
Haas, C. & Steiner, H. Alzheimer disease-γ-secretase: a complex story of GxGD-type presenilin proteases. Trends Cell Biol. 12, 556–562 (2002).
Edbauer, D. et al. Reconstitution of γ-secretase activity. Nat. Cell Biol. 5, 486–488 (2003).
Haass, C. Take five-BACE and the γ-secretase quartet conduct Alzheimer's amyloid β-peptide generation. EMBO J. 23, 483–488 (2004).
Hutton, M., Perez-Tur, J. & Hardy, J. Genetics of Alzheimer's disease. Essays Biochem. 33, 117–131 (1998).
Scheuner, D. et al. Secreted amyloid β-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 2, 864–870 (1996).
Borchelt, D.R. et al. Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron 19, 939–945 (1997).
De Strooper, B. et al. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature 391, 387–390 (1998).
Naruse, S. et al. Effects of PS1 deficiency on membrane protein trafficking in neurons. Neuron 21, 1213–1221 (1998).
Lu, D.C. et al. A second cytotoxic proteolytic pepide derived from amyloid β-protein precursor. Nat. Med. 6, 385–386 (2000).
Stewart, W.F., Kawas, C., Corrada, M. & Metter, E.J. Risk of Alzheimer's disease and duration of NSAID use. Neurology 48, 626–632 (1997).
Weggen, S. et al. A subset of NSAIDs lower amyloidogenic Aβ42 independently of cyclooxygenase activity. Nature 414, 212–216 (2001).
Lipton, S.A. Concepts: turning down but not off—neuroprotection requires a paradigm shift in drug development. Nature 428, 473 (2004).
Kamal, A., Stokin, G.B., Yang, Z., Xia, C.H. & Goldstein, L.S. Axonal transport of amyloid precursor protein is mediated by direct binding to the kinesin light chain subunit of kinesin-I. Neuron 28, 449–459 (2000).
Kamal, A., Almenar-Queralt, A., LeBlanc, J.F., Roberts, E.A. & Goldstein, L.S. Kinesin-mediated axonal transport of a membrane compartment containing β-secretase and presenilin-1 requires APP. Nature 414, 643–648 (2001).
Pigino, G. et al. Alzheimer's presenilin 1 mutations impair kinesin-based axonal transport. J. Neurosci. 23, 4499–4508 (2003).
Selkoe, D.J. Alzheimer's disease is a synaptic failure. Science 298, 789–791 (2002).
Chapman, P.F. et al. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat. Neurosci. 2, 271–276 (1999).
Walsh, D.M. et al. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 416, 535–539 (2002).
Stephan, A., Laroche, S. & Davis, S. Generation of aggregated β-amyloid in the rat hippocampus impairs synaptic transmission and plasticity and causes memory deficits. J. Neurosci. 21, 5703–5714 (2001).
Bush, A.I. et al. A novel zinc(II) binding site modulates the function of the βA4 amyloid protein precursor of Alzheimer's disease. J. Biol. Chem. 268, 16109–16112 (1993).
Bush, A.I. et al. Rapid induction of Alzheimer A β amyloid formation by zinc. Science 265, 1464–1467 (1994).
Lovell, M.A., Robertson, J.D., Teesdale, W.J., Campbell, J.L. & Markesbery, W.R. Copper, iron and zinc in Alzheimer's disease senile plaques. J. Neurol. Sci. 158, 47–52 (1998).
Dong, J. et al. Metal binding and oxidation of amyloid-β within isolated senile plaque cores: Raman microscopic evidence. Biochemistry 42, 2768–2773 (2003).
Opazo, C. et al. Metalloenzyme-like activity of Alzheimer's disease β-amyloid. Cu-dependent catalytic conversion of dopamine, cholesterol, and biological reducing agents to neurotoxic H2O2 . J. Biol. Chem. 277, 40302–40308 (2002).
Bush, A.I. Copper, zinc, and the metallobiology of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 17, 147–150 (2003).
Huang, X. et al. The A β peptide of Alzheimer's disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry 38, 7609–7616 (1999).
Bossy-Wetzel, E. et al. Crosstalk between nitric oxide and zinc pathways to neuronal cell death involving mitochondrial dysfunction and p38-activated K+ channels. Neuron 41, 351–365 (2004).
Bush, A.I. Metal complexing agents as therapies for Alzheimer's disease. Neurobiol. Aging 23, 1031–1038 (2002).
Cherny, R.A. et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron 30, 665–676 (2001).
Ritchie, C.W. et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Aβ amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch. Neurol. 60, 1685–1691 (2003).
Hirai, K. et al. Mitochondrial abnormalities in Alzheimer's disease. J. Neurosci. 21, 3017–3023 (2001).
Anandatheerthavarada, H.K., Biswas, G., Robin, M.A. & Avadhani, N.G. Mitochondrial targeting and a novel transmembrane arrest of Alzheimer's amyloid precursor protein impairs mitochondrial function in neuronal cells. J. Cell Biol. 161, 41–54 (2003).
Casley, C.S. et al. β-Amyloid fragment 25–35 causes mitochondrial dysfunction in primary cortical neurons. Neurobiol. Dis. 10, 258–267 (2002).
Casley, C.S., Canevari, L., Land, J.M., Clark, J.B. & Sharpe, M.A. β-Amyloid inhibits integrated mitochondrial respiration and key enzyme activities. J. Neurochem. 80, 91–100 (2002).
Lustbader, J.W. et al. ABAD directly links Aβ to mitochondrial toxicity in Alzheimer's disease. Science 304, 448–452 (2004).
Xu, J. et al. Dopamine-dependent neurotoxicity of α-synuclein: a mechanism for selective neurodegeneration in Parkinson disease. Nat. Med. 8, 600–606 (2002).
Hardy, J., Cookson, M.R. & Singleton, A. Genes and parkinsonism. Lancet Neurol. 2, 221–228 (2003).
Langston, J.W., Ballard, P., Tetrud, J.W. & Irwin, I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science 219, 979–980 (1983).
Langston, J.W. et al. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 46, 598–605 (1999).
Rose, S. et al. Age-related effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine treatment of common marmosets. Eur. J. Pharmacol. 230, 177–185 (1993).
Irwin, I., DeLanney, L.E. & Langston, J.W. MPTP and aging. Studies in the C57BL/6 mouse. Adv. Neurol. 60, 197–206 (1993).
Ovadia, A., Zhang, Z. & Gash, D.M. Increased susceptibility to MPTP toxicity in middle-aged rhesus monkeys. Neurobiol. Aging 16, 931–937 (1995).
Kaur, D. et al. Genetic or pharmacological iron chelation prevents MPTP-induced neurotoxicity in vivo: a novel therapy for Parkinson's disease. Neuron 37, 899–909 (2003).
Betarbet, R. et al. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat. Neurosci. 3, 1301–1306 (2000).
Polymeropoulos, M.H. et al. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science 276, 2045–2047 (1997).
Kitada, T. et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 392, 605–608 (1998).
Bonifati, V. et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 299, 256–259 (2003).
Valente, E.M. et al. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304, 1158–1160 (2004)
Eriksen, J.L., Dawson, T.M., Dickson, D.W. & Petrucelli, L. Caught in the act. α-Synuclein is the culprit in Parkinson's disease. Neuron 40, 453–456 (2003).
Baptista, M.J., Cookson, M.R. & Miller, D.W. Parkin and α-synuclein: opponent actions in the pathogenesis of Parkinson's disease. Neuroscientist 10, 63–72 (2004).
Sakata, E. et al. Parkin binds the Rpn10 subunit of 26S proteasomes through its ubiquitin-like domain. EMBO Rep. 4, 301–306 (2003).
Cookson, M.R. Parkin's substrates and the pathways leading to neuronal damage. Neuromolecular Med. 3, 1–13 (2003).
Cookson, M.R. et al. RING finger 1 mutations in Parkin produce altered localization of the protein. Hum. Mol. Genet. 12, 2957–2965 (2003).
Imai, Y. et al. An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105, 891–902 (2001).
Petrucelli, L. et al. Parkin protects against the toxicity associated with mutant α-synuclein: proteasome dysfunction selectively affects catecholaminergic neurons. Neuron 36, 1007–1019 (2002).
Staropoli, J.F. et al. Parkin is a component of an SCF-like ubiquitin ligase complex and protects postmitotic neurons from kainate excitotoxicity. Neuron 37, 735–749 (2003).
Darios, F. et al. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum. Mol. Genet. 12, 517–526 (2003).
Yang, Y., Nishimura, I., Imai, Y., Takahashi, R. & Lu, B. Parkin suppresses dopaminergic neuron-selective neurotoxicity induced by Pael-R in Drosophila. Neuron 37, 911–924 (2003).
Tsai, Y.C., Fishman, P.S., Thakor, N.V. & Oyler, G.A. Parkin facilitates the elimination of expanded polyglutamine proteins and leads to preservation of proteasome function. J. Biol. Chem. 278, 22044–22055 (2003).
Greene, J.C. et al. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA 100, 4078–4083 (2003).
Palacino, J.J. et al. Mitochondrial dysfunction and oxidative damage in Parkin-deficient mice. J. Biol. Chem. 279, 18614–18622 (2004).
Chung, K.K. et al. S-Nitrosylation of parkin regulates ubiquitination and compromises parkin's protective function. Science 304, 1328–1331 (2004)
Yao, D., et al. Nitrosative stress linked to sporadic Parkinson's disease: S-nitrosylation of parkin regulates its E3 ligase activity. Proc. Natl. Acad. Sci. USA (2004), in press.
Cookson, M.R. Pathways to parkinsonism. Neuron 37, 7–10 (2003).
Taira, T. et al. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 5, 213–218 (2004).
Honbou, K. et al. The crystal structure of DJ-1, a protein related to male fertility and Parkinson's disease. J. Biol. Chem. 278, 31380–31384 (2003).
Wilson, M.A., Collins, J.L., Hod, Y., Ringe, D. & Petsko, G.A. The 1.1-Å resolution crystal structure of DJ-1, the protein mutated in autosomal recessive early-onset Parkinson's disease. Proc. Natl. Acad. Sci. USA 100, 9256–9261 (2003).
Trottier, Y. et al. Polyglutamine expansion as a pathological epitope in Huntington's disease and four dominant cerebellar ataxias. Nature 378, 403–406 (1995).
DiFiglia, M. et al. Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron 14, 1075–1081 (1995).
Panov, A.V. et al. Early mitochondrial calcium defects in Huntington's disease are a direct effect of polyglutamines. Nat. Neurosci. 5, 731–736 (2002).
Young, A.B. Huntingtin in health and disease. J. Clin. Invest. 111, 299–302 (2003).
Harjes, P. & Wanker, E.E. The hunt for huntingtin function: interaction partners tell many different stories. Trends Biochem. Sci. 28, 425–433 (2003).
Li, H., Gu, X., Dawson, V.L. & Dawson, T.M. Identification of calcium- and nitric oxide–regulated genes by differential analysis of library expression (DAzLE). Proc. Natl. Acad. Sci. USA 101, 647–652 (2004).
Andrade, M.A. & Bork, P. HEAT repeats in the Huntington's disease protein. Nat. Genet. 11, 115–116 (1995).
Sittler, A. et al. SH3GL3 associates with the Huntingtin exon 1 protein and promotes the formation of polygln-containing protein aggregates. Mol. Cell 2, 427–436 (1998).
Sun, Y., Savanenin, A., Reddy, P.H. & Liu, Y.F. Polyglutamine-expanded huntingtin promotes sensitization of N-methyl-d-aspartate receptors via post-synaptic density 95. J. Biol. Chem. 276, 24713–24718 (2001).
Wanker, E.E. et al. HIP-I: a huntingtin interacting protein isolated by the yeast two-hybrid system. Hum. Mol. Genet. 6, 487–495 (1997).
Kalchman, M.A. et al. HIP1, a human homologue of S. cerevisiae Sla2p, interacts with membrane-associated huntingtin in the brain. Nat. Genet. 16, 44–53 (1997).
Li, X.J. et al. A huntingtin-associated protein enriched in brain with implications for pathology. Nature 378, 398–402 (1995).
Singaraja, R.R. et al. HIP14, a novel ankyrin domain-containing protein, links huntingtin to intracellular trafficking and endocytosis. Hum. Mol. Genet. 11, 2815–2828 (2002).
Feany, M.B. & La Spada, A.R. Polyglutamines stop traffic: axonal transport as a common target in neurodegenerative diseases. Neuron 40, 1–2 (2003).
Li, H., Li, S.H., Yu, Z.X., Shelbourne, P. & Li, X.J. Huntingtin aggregate-associated axonal degeneration is an early pathological event in Huntington's disease mice. J. Neurosci. 21, 8473–8481 (2001).
Gunawardena, S. et al. Disruption of axonal transport by loss of huntingtin or expression of pathogenic polyQ proteins in Drosophila. Neuron 40, 25–40 (2003).
Steffan, J.S. et al. Histone deacetylase inhibitors arrest polyglutamine-dependent neurodegeneration in Drosophila. Nature 413, 739–743 (2001).
Dunah, A.W. et al. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington's disease. Science 296, 2238–2243 (2002).
Obrietan, K. & Hoyt, K.R. CRE-mediated transcription is increased in Huntington's disease transgenic mice. J. Neurosci. 24, 791–796 (2004).
Sugars, K.L., Brown, R., Cook, L.J., Swartz, J. & Rubinsztein, D.C. Decreased cAMP response element–mediated transcription: an early event in exon 1 and full-length cell models of Huntington's disease that contributes to polyglutamine pathogenesis. J. Biol. Chem. 279, 4988–4999 (2004).
Sipione, S. & Cattaneo, E. Modeling Huntington's disease in cells, flies, and mice. Mol. Neurobiol. 23, 21–51 (2001).
Rubinsztein, D.C. Lessons from animal models of Huntington's disease. Trends Genet. 18, 202–209 (2002).
Rosen, D.R. et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362, 59–62 (1993).
Hough, M.A. et al. Dimer destabilization in superoxide dismutase may result in disease-causing properties: structures of motor neuron disease mutants. Proc. Natl. Acad. Sci. USA 101, 5976–5981 (2004).
Koo, E.H., Lansbury, P.T., Jr. & Kelly, J.W. Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proc. Natl. Acad. Sci. USA 96, 9989–9990 (1999).
Ray, S.S. & Lansbury, P.T., Jr. A possible therapeutic target for Lou Gehrig's disease. Proc. Natl. Acad. Sci. USA 101, 5701–5702 (2004).
Clement, A.M. et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science 302, 113–117 (2003).
Kawahara, Y. et al. Glutamate receptors: RNA editing and death of motor neurons. Nature 427, 801 (2004).
Lipton, S.A. Sporadic ALS: blame it on the editor. Nat. Med. 10, 347 (2004).
Acknowledgements
We thank Eliezer Masliah and Mark Barsoum for providing histological images, Huaxi Xu for reading the manuscript and our colleagues for helpful discussions. This work was supported by NIH grants R01 NS44314 and R01 NS047456 (to E.B-W.) and P01 HD29587, R01 EY05477, R01 EY09024, R01 NS43242, R01 NS44326 and R01 NS41207 (to S.A.L.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
S.A.L. is the named inventor on patents for the drug memantine to treat neurodegenerative disorders. Under agreements between Children's Hospital/Harvard Medical School and Forest Laboratories/Merz & Co./Neurobiological Technologies, Inc., S.A.L. is thus entitled to a share of sales royalties received by the hospital. S.A.L. owns no stock in these companies. The terms of this arrangement are being managed by Harvard Medical School in accordance with its conflict-of-interest policies.
Rights and permissions
About this article
Cite this article
Bossy-Wetzel, E., Schwarzenbacher, R. & Lipton, S. Molecular pathways to neurodegeneration. Nat Med 10 (Suppl 7), S2–S9 (2004). https://doi.org/10.1038/nm1067
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1067
This article is cited by
-
Lead-exposure associated miRNAs in humans and Alzheimer’s disease as potential biomarkers of the disease and disease processes
Scientific Reports (2022)
-
Dietary restriction ameliorates TBI-induced phenotypes in Drosophila melanogaster
Scientific Reports (2022)
-
Epigenetic Changes and Its Intervention in Age-Related Neurodegenerative Diseases
Cellular and Molecular Neurobiology (2022)
-
Quantitative metabolomics of saliva using proton NMR spectroscopy in patients with Parkinson’s disease and healthy controls
Neurological Sciences (2020)
-
The Effect of Sialic Acid on the Expression of miR-218, NF-kB, MMP-9, and TIMP-1
Biochemical Genetics (2020)