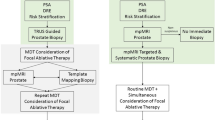
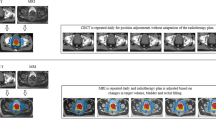
Abstract
This Review highlights current areas of controversy and development in the field of transperineal permanent prostate seed implantation brachytherapy (PPI), in particular the technological evolution of PPI treatment planning that has led to intra-operative treatment planning and execution, the use of MRI spectroscopy and ultrasonography to target intraprostatic tumor foci, and the introduction of 131Cs as a new PPI isotope. Here we present a comprehensive review of mature data for PPI monotherapy and PPI combined with supplemental external beam radiation therapy, and a critical discussion of issues pertinent to supplemental EBRT. We also present our current policies in the treatment of prostate cancer at the University of California, San Francisco.
Key Points
-
For patients with low risk prostate cancer, permanent prostate seed implantation brachytherapy (PPI) monotherapy achieves durable long-term biochemical control
-
Intra-operative treatment planning might replace pre-planning with the potential for better prostate dosimetric outcomes
-
Favorable long-term results for intermediate- and high-risk patients have been reported for combined PPI and external beam radiation therapy, and are the subject of a current randomized trial
-
MRI spectroscopy and advanced ultrasound techniques potentially allow for dose escalation of tumor foci within the gland
-
MRI spectroscopy provides biological confirmation of gland atrophy following radiation therapy
-
The main difference in iodine and palladium isotopes for PPI lie in their urinary adverse effect profile, and cesium is a new isotope emerging into clinical practice for PPI
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Nag S et al. (1999) American Brachytherapy Society (ABS) recommendations for transperineal permanent brachytherapy of prostate cancer. Int J Radiat Oncol Biol Phys 44: 789–799
Roach M III et al. (2006) Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 65: 965–974
D'Amico AV et al. (1998) Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 280: 969–974
Sharkey J et al. (2005) 103Pd brachytherapy versus radical prostatectomy in patients with clinically localized prostate cancer: a 12-year experience from a single group practice. Brachytherapy 4: 34–44
Kupelian PA et al. (2002) Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: a large single-institution experience with radical prostatectomy and external-beam radiotherapy. J Clin Oncol 20: 3376–3385
D'Amico AV et al. (2003) Comparing PSA outcome after radical prostatectomy or magnetic resonance imaging-guided partial prostatic irradiation in select patients with clinically localized adenocarcinoma of the prostate. Urology 62: 1063–1067
Brachman DG et al. (2000) Failure-free survival following brachytherapy alone or external beam irradiation alone for T1–2 prostate tumors in 2,222 patients: results from a single practice. Int J Radiat Oncol Biol Phys 48: 111–117
Stokes SH (2000) Comparison of biochemical disease-free survival of patients with localized carcinoma of the prostate undergoing radical prostatectomy, transperineal ultrasound-guided radioactive seed implantation, or definitive external beam irradiation. Int J Radiat Oncol Biol Phys 47: 129–136
National Comprehensive Cancer Network (2007) NCCN Clinical Practice in Oncology Guidelines for Prostate Cancer v.2.2007 [http://www.nccn.org/professionals/physician_gls/PDF/prostate.pdf] (accessed 9 October 2007)
Morton GC (2005) The emerging role of high-dose-rate brachytherapy for prostate cancer. Clin Oncol (R Coll Radiol) 17: 219–227
Keyes M et al. (2006) Decline in urinary retention incidence in 805 patients after prostate brachytherapy: the effect of learning curve? Int J Radiat Oncol Biol Phys 64: 825–834
Nag S et al. (2001) Intraoperative planning and evaluation of permanent prostate brachytherapy: report of the American Brachytherapy Society. Int J Radiat Oncol Biol Phys 51: 1422–1430
Stock RG et al. (2000) Postimplant dosimetry for (125)I prostate implants: definitions and factors affecting outcome. Int J Radiat Oncol Biol Phys 48: 899–906
Zelefsky MJ et al. (2007) Multi-institutional analysis of long-term outcome for stages T1–T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys 67: 327–333
Ash D et al. (2005) The impact of hormone therapy on post-implant dosimetry and outcome following iodine-125 implant monotherapy for localised prostate cancer. Radiother Oncol 75: 303–306
Wilkinson DA et al. (2000) Dosimetric comparison of pre-planned and OR-planned prostate seed brachytherapy. Int J Radiat Oncol Biol Phys 48: 1241–1244
Shanahan TG et al. (2002) A comparison of permanent prostate brachytherapy techniques: preplan vs hybrid interactive planning with postimplant analysis. Int J Radiat Oncol Biol Phys 53: 490–496
Beyer DC et al. (2000) Real-time optimized intraoperative dosimetry for prostate brachytherapy: a pilot study. Int J Radiat Oncol Biol Phys 48: 1583–1589
Zelefsky MJ et al. (2003) Improved conformality and decreased toxicity with intraoperative computer-optimized transperineal ultrasound-guided prostate brachytherapy. Int J Radiat Oncol Biol Phys 55: 956–963
Potters L et al. (2003) Toward a dynamic real-time intraoperative permanent prostate brachytherapy methodology. Brachytherapy 2: 172–180
Raben A et al. (2004) Prostate seed implantation using 3D-computer assisted intraoperative planning vs a standard look-up nomogram: improved target conformality with reduction in urethral and rectal wall dose. Int J Radiat Oncol Biol Phys 60: 1631–1638
French D et al. (2005) Computing intraoperative dosimetry for prostate brachytherapy using TRUS and fluoroscopy. Acad Radiol 12: 1262–1272
Todor DA et al. (2003) Intraoperative dynamic dosimetry for prostate implants. Phys Med Biol 48: 1153–1171
American Society for Therapeutic Radiology and Oncology Consensus Panel (1997) Consensus statement: guidelines for PSA following radiation therapy. Int J Radiat Oncol Biol Phys 37: 1035–1041
Thames H et al. (2003) Comparison of alternative biochemical failure definitions based on clinical outcome in 4,839 prostate cancer patients treated by external beam radiotherapy between 1986 and 1995. Int J Radiat Oncol Biol Phys 57: 929–943
Kuban DA et al. (2006) Comparison of biochemical failure definitions for permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 65: 1487–1493
Pickles T (2006) Prostate-specific antigen (PSA) bounce and other fluctuations: which biochemical relapse definition is least prone to PSA false calls? An analysis of 2,030 men treated for prostate cancer with external beam or brachytherapy with or without adjuvant androgen deprivation therapy. Int J Radiat Oncol Biol Phys 64: 1355–1359
Zelefsky MJ and Whitmore WF Jr (1997) Long-term results of retropubic permanent 125iodine implantation of the prostate for clinically localized prostatic cancer. J Urol 158: 23–29
Kuban DA et al. (1989) I-125 interstitial implantation for prostate cancer. What have we learned 10 years later? Cancer 63: 2415–2420
Partin AW et al. (1997) Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA 277: 1445–1451
Kattan MW et al. (1998) A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst 90: 766–771
Davis BJ et al. (1999) The radial distance of extraprostatic extension of prostate carcinoma: implications for prostate brachytherapy. Cancer 85: 2630–2637
Sohayda C et al. (2000) Extent of extracapsular extension in localized prostate cancer. Urology 55: 382–386
Febles C and Valicenti RK (2004) Combining external beam radiotherapy with prostate brachytherapy: issues and rationale. Urology 64: 855–861
Merrick GS et al. (2006) Permanent prostate brachytherapy: is supplemental external-beam radiation therapy necessary? Oncology (Williston Park) 20: 514–522
Roach M III (2003) “Supplemental beam” and prostate brachytherapy: a simple answer to a complicated question? Int J Radiat Oncol Biol Phys 55: 1162–1163
Roach M III et al. (2003) Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol 21: 1904–1911
Roach M III et al. (2006) Whole-pelvis, “mini-pelvis”, or prostate-only external beam radiotherapy after neoadjuvant and concurrent hormonal therapy in patients treated in the Radiation Therapy Oncology Group 9413 trial. Int J Radiat Oncol Biol Phys 66: 647–653
Merrick GS et al. (2006) Brachytherapy in men aged ≤54 years with clinically localized prostate cancer. BJU Int 98: 324–328
Blasko JC et al. (2000) The role of external beam radiotherapy with I-125/Pd-103 brachytherapy for prostate carcinoma. Radiother Oncol 57: 273–278
Sylvester JE et al. (2007) 15-year biochemical relapse free survival in clinical Stage T1–T3 prostate cancer following combined external beam radiotherapy and brachytherapy; Seattle experience. Int J Radiat Oncol Biol Phys 67: 57–64
Merrick GS et al. (2002) Biochemical outcome for hormone-naive intermediate-risk prostate cancer managed with permanent interstitial brachytherapy and supplemental external beam radiation. Brachytherapy 1: 95–101
Merrick GS et al. (2004) Permanent interstitial brachytherapy in younger patients with clinically organ-confined prostate cancer. Urology 64: 754–759
Dattoli M et al. (2007) Long-term prostate cancer control using palladium-103 brachytherapy and external beam radiotherapy in patients with a high likelihood of extracapsular cancer extension. Urology 69: 334–337
Pollack A et al. (2002) Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys 53: 1097–1105
Zietman AL et al. (2005) Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA 294: 1233–1239
Peeters ST et al. (2006) Dose-response in radiotherapy for localized prostate cancer: results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol 24: 1990–1996
Dearnaley DP et al. (2007) Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol 8: 475–487
Sathya JR et al. (2005) Randomized trial comparing iridium implant plus external-beam radiation therapy with external-beam radiation therapy alone in node-negative locally advanced cancer of the prostate. J Clin Oncol 23: 1192–1199
Hoskin PJ et al. (2007) High dose rate brachytherapy in combination with external beam radiotherapy in the radical treatment of prostate cancer: initial results of a randomised phase three trial. Radiother Oncol 84: 114–120
Choi S et al. (2004) Treatment margins predict biochemical outcomes after prostate brachytherapy. Cancer J 10: 175–180
Stone NN et al. (2005) Intermediate term biochemical-free progression and local control following 125iodine brachytherapy for prostate cancer. J Urol 173: 803–807
Beyer DC et al. (2005) Impact of short course hormonal therapy on overall and cancer specific survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 61: 1299–1305
Merrick GS et al. (2006) Androgen-deprivation therapy does not impact cause-specific or overall survival after permanent prostate brachytherapy. Int J Radiat Oncol Biol Phys 65: 669–677
Potters L et al. (2000) Examining the role of neoadjuvant androgen deprivation in patients undergoing prostate brachytherapy. J Clin Oncol 18: 1187–1192
Comet-Batlle J et al. (2003) The value of endorectal MRI in the early diagnosis of prostate cancer. Eur Urol 44: 201–207
Hricak H et al. (1994) Carcinoma of the prostate gland: MR imaging with pelvic phased-array coils versus integrated endorectal–pelvic phased-array coils. Radiology 193: 703–709
Kurhanewicz J et al. (2000) The prostate: MR imaging and spectroscopy. Present and future. Radiol Clin North Am 38: 115–138
Coakley FV et al. (2004) Endorectal MR imaging and MR spectroscopic imaging for locally recurrent prostate cancer after external beam radiation therapy: preliminary experience. Radiology 233: 441–448
DiBiase SJ et al. (2002) Magnetic resonance spectroscopic imaging-guided brachytherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 52: 429–438
Mizowaki T et al. (2002) Towards integrating functional imaging in the treatment of prostate cancer with radiation: the registration of the MR spectroscopy imaging to ultrasound/CT images and its implementation in treatment planning. Int J Radiat Oncol Biol Phys 54: 1558–1564
Pouliot J et al. (2004) Inverse planning for HDR prostate brachytherapy used to boost dominant intraprostatic lesions defined by magnetic resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys 59: 1196–1207
Zaider M et al. (2000) Treatment planning for prostate implants using magnetic-resonance spectroscopy imaging. Int J Radiat Oncol Biol Phys 47: 1085–1096
Zelefsky MJ et al. (2000) Intraoperative conformal optimization for transperineal prostate implantation using magnetic resonance spectroscopic imaging. Cancer J 6: 249–255
Pickett B et al. (2004) Time to metabolic atrophy after permanent prostate seed implantation based on magnetic resonance spectroscopic imaging. Int J Radiat Oncol Biol Phys 59: 665–673
Ellis RJ et al. (2007) Biochemical disease-free survival rates following definitive low-dose-rate prostate brachytherapy with dose escalation to biologic target volumes identified with SPECT/CT capromab pendetide. Brachytherapy 6: 16–25
Feleppa EJ et al. (2000) Three-dimensional ultrasound analyses of the prostate. Mol Urol 4: 133–139
Feleppa EJ et al. (2004) Recent developments in tissue-type imaging (TTI) for planning and monitoring treatment of prostate cancer. Ultrason Imaging 26: 163–172
Czarnota GJ et al. (1999) Ultrasound imaging of apoptosis: high-resolution non-invasive monitoring of programmed cell death in vitro, in situ and in vivo . Br J Cancer 81: 520–527
Czarnota GJ et al. (2002) Ultrasound imaging of apoptosis. DNA-damage effects visualized. Methods Mol Biol 203: 257–277
Kolios MC et al. (2002) Ultrasonic spectral parameter characterization of apoptosis. Ultrasound Med Biol 28: 589–597
Czarnota GJ (2005) Role of ultrasound in the detection of apoptosis. Eur J Nucl Med Mol Imaging 32: 622
Chu W et al. (2007) Functional imaging of apoptosis in human tumours with high frequency ultrasound imaging and spectroscopy. Presented at the American Institute of Ultrasound in Medicine Annual Meeting: 2007, March 15–18, New York, NY
Wallner K et al. (2002) I-125 versus Pd-103 for low-risk prostate cancer: morbidity outcomes from a prospective randomized multicenter trial. Cancer J 8: 67–73
Herstein A et al. (2005) I-125 versus Pd-103 for low-risk prostate cancer: long-term morbidity outcomes from a prospective randomized multicenter controlled trial. Cancer J 11: 385–389
Cha CM et al. (1999) Isotope selection for patients undergoing prostate brachytherapy. Int J Radiat Oncol Biol Phys 45: 391–395
Stock RG et al. (2006) Biologically effective dose values for prostate brachytherapy: effects on PSA failure and posttreatment biopsy results. Int J Radiat Oncol Biol Phys 64: 527–533
Lawton CA et al. (2007) Results of a phase II trial of transrectal ultrasound-guided permanent radioactive implantation of the prostate for definitive management of localized adenocarcinoma of the prostate (radiation therapy oncology group 98-05). Int J Radiat Oncol Biol Phys 67: 39–47
Martin AG et al. (2007) Permanent prostate implant using high activity seeds and inverse planning with fast simulated annealing algorithm: a 12-year Canadian experience. Int J Radiat Oncol Biol Phys 67: 334–341
Torres-Roca JF et al. (2006) Treatment of intermediate-risk prostate cancer with brachytherapy without supplemental pelvic radiotherapy: a review of the H Lee Moffitt Cancer Center experience. Urol Oncol 24: 384–390
Potters L et al. (2005) 12-year outcomes following permanent prostate brachytherapy in patients with clinically localized prostate cancer. J Urol 173: 1562–1566
Kollmeier MA et al. (2003) Biochemical outcomes after prostate brachytherapy with 5-year minimal follow-up: importance of patient selection and implant quality. Int J Radiat Oncol Biol Phys 57: 645–653
Kwok Y et al. (2002) Risk group stratification in patients undergoing permanent (125)I prostate brachytherapy as monotherapy. Int J Radiat Oncol Biol Phys 53: 588–594
Grimm PD et al. (2001) 10-year biochemical (prostate-specific antigen) control of prostate cancer with (125)I brachytherapy. Int J Radiat Oncol Biol Phys 51: 31–40
Zelefsky MJ et al. (1998) Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys 41: 491–500
Lee WR et al. (2007) Late toxicity and biochemical recurrence after external-beam radiotherapy combined with permanent-source prostate brachytherapy: analysis of Radiation Therapy Oncology Group study 0019. Cancer 109: 1506–1512
Critz FA and Levinson K (2004) 10-year disease-free survival rates after simultaneous irradiation for prostate cancer with a focus on calculation methodology. J Urol 172: 2232–2238
Lederman GS et al. (2001) Retrospective stratification of a consecutive cohort of prostate cancer patients treated with a combined regimen of external-beam radiotherapy and brachytherapy. Int J Radiat Oncol Biol Phys 49: 1297–1303
Acknowledgements
We would like to thank Dr J Kurhanewicz from the University of California, San Francisco for his expertise and help in generating the MRI/MRSI figures. Charles P Vega, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Sahgal, A., Roach, M. Permanent prostate seed brachytherapy: a current perspective on the evolution of the technique and its application. Nat Rev Urol 4, 658–670 (2007). https://doi.org/10.1038/ncpuro0971
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpuro0971