Abstract
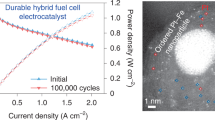
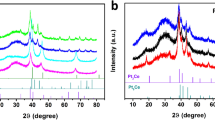
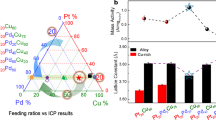
The widespread use of low-temperature polymer electrolyte membrane fuel cells for mobile applications will require significant reductions in the amount of expensive Pt contained within their cathodes, which drive the oxygen reduction reaction (ORR). Although progress has been made in this respect, further reductions through the development of more active and stable electrocatalysts are still necessary. Here we describe a new set of ORR electrocatalysts consisting of Pd or Pt alloyed with early transition metals such as Sc or Y. They were identified using density functional theory calculations as being the most stable Pt- and Pd-based binary alloys with ORR activity likely to be better than Pt. Electrochemical measurements show that the activity of polycrystalline Pt3Sc and Pt3Y electrodes is enhanced relative to pure Pt by a factor of 1.5–1.8 and 6–10, respectively, in the range 0.9–0.87 V.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gasteiger, H. A., Kocha, S. S., Somappli, B. & Wagner, F. T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl. Catal. B-Environ. 56, 9–35 (2005).
Stamenkovic, V. et al. Improved oxygen reduction activity on Pt3Ni(111) via increased surface site availability. Science 315, 493–497 (2007).
Koh, S. & Strasser, P. Electrocatalysis on bimetallic surfaces: modifying catalytic reactivity for oxygen reduction by voltammetric surface dealloying. J. Amer. Chem. Soc. 129, 12624–12625 (2007).
Chen, S. et al. Origin of oxygen reduction reaction activity on “Pt3Co” nanoparticles: atomically resolved chemical compositions and structures. J. Phys. Chem. C 113, 1109–1125 (2009).
Wakisaka, W., Suzuki, H., Mitsui, S., Uchida, H. & Watanabe, M. Increased oxygen coverage at Pt-Fe alloy cathode for the enhanced oxygen reduction reaction studied by EC-XPS. J. Phys. Chem. C 112, 2750–2755 (2008).
Stamenkovic, V. et al. Changing the activity of electrocatalysts for oxygen reduction by tuning the surface electronic structure. Angew. Chem. Int. Ed. 45, 2897–2901 (2006).
Paulus, U. A. et al. Oxygen reduction on high surface area Pt-based alloy catalysts in comparison to well defined smooth bulk alloy electrodes. Electrochim. Acta 47, 3787–3798 (2002).
Paulus, U. A. et al. Oxygen reduction on Carbon-Supported Pt-Ni and Pt-Co Alloy Catalysts. J. Phys. Chem. B 106, 4181–4191 (2002).
Mayrhofer, K. J. J., Juhart, V., Hartl, K., Hanzlik, M. & Arenz, M. Adsorbate-induced surface segregation for core–shell nanocatalysts. Angew. Chem. Int. Ed. 48, 3529–3531 (2009).
Koh, S., Leisch, J., Toney, M. F. & Strasser, P. Structure–activity–stability relationships of Pt–Co alloy electrocatalysts in gas-diffusion electrode layers. J. Phys. Chem. C 111, 3744–3752 (2007).
Koh, S., Toney, M. F. & Strasser, P. Activity–stability relationships of ordered and disordered alloy phases of Pt3Co electrocatalysts for the oxygen reduction reaction (ORR). Electrochim. Acta 52, 2765–2774 (2007).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Nørskov, J. K, Bligaard, T., Rossmeisl, J. & Christensen, C. H. Towards the computational design of solid catalysts. Nature Chem. 1, 37–46 (2009).
Strasser, P., Fan, Q., Devenney, M. & Weinberg, W. H. High throughput experimental and theoretical screening of materials—a comparative study of search strategies for new fuel cell anode catalysts. J. Phys. Chem B 107, 11013–11021 (2003).
Skulason, E. et al. Density functional theory calculations for the hydrogen evolution reaction in an electrochemical double layer on the Pt(111) electrode. Phys. Chem. Chem. Phys. 9, 3241–3250 (2007).
Janik, M. J., Taylor, C. D. & Neurock, M. First-principles analysis of the initial electroreduction steps of oxygen over Pt(111). J. Electrochem. Soc. 156, B126–B135 (2009).
Greeley, J., Rossmeisl, J., Hellman, A. & Nørskov, J. K. Theoretical trends in particle size effects for the oxygen reduction reaction. Z. Phys. Chem. 221, 1209–1220 (2007).
Stamenkovic, V. R. et al. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nature Mater. 6, 241–247 (2007).
Rossmeisl, J., Logadottir, A. & Nørskov, J. K. Electrolysis of water on (oxidized) metal surfaces. Chem. Phys. 319, 178–184 (2005).
Abild-Pedersen, F. et al. Scaling properties of adsorption energies for hydrogen-containing molecules on transition-metal surfaces. Phys. Rev. Lett. 99, 016105 (2007).
Stamenkovic, V. R., Mun, B. S., Mayrhofer, K. J. & Ross, P. N., Markovic, N. M. Effect of surface composition on electronic structure, stability, and electrocatalytic properties of Pt-transition metal alloys: Pt-skin versus Pt-skeleton surfaces. J. Am. Chem. Soc. 128, 8813–8819 (2006).
Johannesson G. H. et al. Combined electronic structure and evolutionary search approach to materials design. Phys. Rev. Lett. 88, 255506 (2002).
Bligaard, T. et al. Pareto-optimal alloys. Appl. Phys. Lett. 83, 4527–4529 (2003).
Ruban, A. V., Skriver, H. L. & Nørskov, J. K. Crystal-structure contribution to the solid solubility in transition metal alloys. Phys. Rev. Lett. 80, 1240 (1998).
Stamenkovic, V. R., Schmidt, T. J., Ross, P. N. & Markovic, N. M. Surface composition effects in electrocatalysis: kinetics of oxygen reduction on well-defined Pt3Ni and Pt3Co alloy surfaces J. Phys. Chem. B 106, 11970–11979 (2002).
Neyerlin, K. C., Srivastava, R., Yu, C. & Strasser, P. Electrochemical activity and stability of dealloyed Pt–Cu and Pt-Cu-Co electrocatalysts for the oxygen reduction reaction (ORR). J. Power Sources 186, 261–267 (2009).
Ball, S. C., Hudson, S. L., Theobald, B. R. C. & Thompsett, D. PtCo, a durable catalyst for automotive proton electrolyte membrane fuel cells? ECS Transactions 11, 1267–1278 (2007).
Greeley, J. & Nørskov, J. K. Combinatorial density functional theory-based screening of surface alloys for the oxygen reduction reaction. J. Phys. Chem. C 113, 4932–4939 (2009).
Rossmeisl, J., Qu, Z.-W., Zhu, H., Kroes, G.-J. & Nørskov, J. K. Electrolysis of water on oxide surfaces. J. Electroanal. Chem. 607, 83–89 (2007).
Takasu, Y., Yoshinaga, N. & Sugimoto, W. Oxygen reduction behavior of RuO2/Ti, IrO2/Ti and IrM (M: Ru, Mo, W, V) Ox/Ti binary oxide electrodes in a sulfuric acid solution. Electrochem. Commun. 10, 668–672 (2008).
Mano, N., Soukharev, V. & Heller, A. A laccase-wiring redox hydrogel for efficient catalysis of O2 electroreduction. J. Phys. Chem. B 110, 11180–11187 (2006).
Lefèvre, M., Proietti, E., Jaouen, F. & Dodelet, J.-P. Iron-based catalysts with improved oxygen reduction activity in polymer electrolyte fuel cells. Science 324, 71–74 (2009).
Gasteiger, H. A. & Markovic, N. M. Just a dream—or future reality? Science 324, 48 (2009).
Zhang, J. L, Vukmirovic, M. B., Xu, Y., Mavrikakis, M. & Adzic, R. R. Controlling the catalytic activity of platinum-monolayer electrocatalysts for oxygen reduction with different substrates. Angew. Chem. Int. Ed. 44, 2132–2135 (2005).
Rossmeisl, J., Karlberg, G. S., Jaramillo, T. F. & Nørskov, J. K. Steady state oxygen reduction and cyclic voltammetry. Faraday Discuss. 140, 337–346 (2008).
Karlberg, G. S., Rossmeisl, J. & Nørskov, J. K. Estimations of electric field effects on the oxygen reduction reaction based on the density functional theory. Phys. Chem. Chem. Phys. 9, 5158–5161 (2007).
Acknowledgements
J.G. and T.F.J. are both recipients of H. C. Ørsted Postdoctoral Fellowships from the Technical University of Denmark. Funding by the Danish Council for Technology and Innovation's FTP program and by the Danish Strategic Research Council's HyCycle program is gratefully acknowledged. The Center for Atomic-scale Materials Design is supported by the Lundbeck Foundation. The Center for Individual Nanoparticle Functionality is supported by the Danish National Research Foundation.
Author information
Authors and Affiliations
Contributions
J.G., H.A.H., J.R. and J.K.N. contributed to the computational work in this paper. I.E.L.S., A.S.B., T.P.J., T.F.J., and I.C. contributed to the experimental work.
Corresponding author
Ethics declarations
Competing interests
The Technical University of Denmark (DTU) has filed a patent with the authors named as the inventors.
Supplementary information
Supplementary information
Supplementary information (PDF 1002 kb)
Rights and permissions
About this article
Cite this article
Greeley, J., Stephens, I., Bondarenko, A. et al. Alloys of platinum and early transition metals as oxygen reduction electrocatalysts. Nature Chem 1, 552–556 (2009). https://doi.org/10.1038/nchem.367
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.367