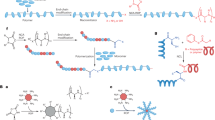
Abstract
Macromolecular antimicrobial agents such as cationic polymers and peptides have recently been under an increased level of scrutiny because they can combat multi-drug-resistant microbes. Most of these polymers are non-biodegradable and are designed to mimic the facially amphiphilic structure of peptides so that they may form a secondary structure on interaction with negatively charged microbial membranes. The resulting secondary structure can insert into and disintegrate the cell membrane after recruiting additional polymer molecules. Here, we report the first biodegradable and in vivo applicable antimicrobial polymer nanoparticles synthesized by metal-free organocatalytic ring-opening polymerization of functional cyclic carbonate. We demonstrate that the nanoparticles disrupt microbial walls/membranes selectively and efficiently, thus inhibiting the growth of Gram-positive bacteria, methicillin-resistant Staphylococcus aureus (MRSA) and fungi, without inducing significant haemolysis over a wide range of concentrations. These biodegradable nanoparticles, which can be synthesized in large quantities and at low cost, are promising as antimicrobial drugs, and can be used to treat various infectious diseases such as MRSA-associated infections, which are often linked with high mortality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Hancock, R. E. W. & Sahl, H. G. Antimicrobial and host–defence peptides as new anti-infective therapeutic strategies. Nature Biotechnol. 24, 1551–1557 (2006).
Radzishevsky, I. S. et al. Improved antimicrobial peptides based on acyl-lysine oligomers. Nature Biotechnol. 25, 657–659 (2007).
Brogden, K. A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria. Nature Rev. Microbiol. 3, 238–250 (2005).
Boman, H. G. Antibacterial peptides: key components needed in immunity. Cell 65, 205–207 (1991).
Lehrer, R. I. & Ganz, T. Antimicrobial peptides in mammalian and insect host defense. Curr. Opin. Immunol. 11, 23–27 (1999).
Hoffmann, J. A., Kafatos, F. C., Janeway, C. A. & Ezekowitz, R. A. Phylogenetic perspectives in innate immunity. Science 284, 1313–1318 (1999).
Oren, Z. et al. Structures and mode of membrane interaction of a short α helical lytic peptide and its diastereomer determined by NMR, FTIR, and fluorescence spectroscopy. Eur. J. Biochem. 269, 3869–3880 (2002).
Oren, Z. & Shai, Y. Cyclization of a cytolytic amphipathic α-helical peptide and its diastereomer: effect on structure, interaction with model membranes, and biological function. Biochemistry 39, 6103–6114 (2000).
Shai, Y. Mode of action of membrane-active antimicrobial peptides. Biopolymers (Peptide Science) 66, 236–248 (2002).
Fernandez-Lopez, S. et al. Antibacterial agents based on the cyclic D,L-α-peptide architecture. Nature 412, 452–455 (2001).
Jerold Gordon, Y. & Romanowski, E. G. A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr. Eye Res. 30, 505–515 (2005).
Lienkamp, K. et al. Antimicrobial polymers prepared by ROMP with unprecedented selectivity: a molecular construction kit approach. J. Am. Chem. Soc. 130, 9836–9843 (2008).
AL-Badri, Z. M. et al. Investigating the effect if increasing charge density on the hemolytic activity of synthetic antimicrobial polymers. Biomacromolecules 9, 2805–2810 (2008).
Ilker, M. F., Nüsslein, K., Tew, G. N. & Coughlin, E. B. Tuning the hemolytic and antimicrobial activities of amphiphilic polynorbornene derivatives. J. Am. Chem. Soc. 126, 15870–15875 (2004).
Kenawy, E.-R., Worley, S. D. & Broughton, R. The chemistry and application of antimicrobial polymers: a state-of-the-art review. Biomacromolecules 8, 1359–1384 (2007).
Kuroda, K. & DeGrado, W. F. Amphiphilic polymethacrylate derivatives as antimicrobial agents. J. Am. Chem. Soc. 127, 4128–4129 (2005).
Ivanov, I. et al. Characterization of nonbiological antimicrobial polymers in aqueous solution and at water–lipid interfaces from all-atom molecular dynamics. J. Am. Chem. Soc. 128, 1778–1779 (2006).
Tew, G. N. et al. De novo design of biomimetic antimicrobial polymers. Proc. Natl Acad. Sci. USA 99, 5110–5114 (2002).
Mowery, B. P. et al. Mimicry of antimicrobial host–defense peptides by random copolymers. J. Am. Chem. Soc. 129, 15474–15476 (2007).
Sambhy, V., Peterson, B. R. & Sen, A. Antibacterial and hemolytic activities of pyridinium polymers as a function of the spatial relationship between the positive charge and the pendant alkyl tail. Angew. Chem. Int. Ed. 47, 1250–1254 (2008).
Chan, D. I., Prenner, E. J. & Vogel, H. J. Tryptophan- and arginine-rich antimicrobial peptides: structures and mechanisms of action. Biochim. Biophys. Acta Biomembranes 1758, 1184–1202 (2006).
Liu, L. H. et al. Self-assembled cationic peptide nanoparticles as an efficient antimicrobial agent. Nature Nanotech. 4, 457–463 (2009).
Mei, H., Zhong, Z., Long, F. & Zhuo, R. Synthesis and characterization of novel glycol-derived polycarbonates with pendant hydroxyl groups. Macromol. Rapid Commun. 27, 1894–1899 (2006).
Zhu, K. J., Hendren, R. W., Jensen, K. C. & Pitt, G. Synthesis, properties, and biodegradation of poly(1,3-trimethylene carbonate). Macromolecules 24, 1736–1740 (1991).
Watanabe, J., Kotera, H. & Akashi, M. Reflective interfaces of poly(trimethylene carbonate)-based polymers: enzymatic degradation and selective adsorption. Macromolecules 40, 8731–8736 (2007).
Edlund, U., Albertsson, A.-C., Singh, S. K., Fogelberg, I. & Lundgren, B. O. Sterilization, storage stability and in vivo biocompatibility of poly(trimethylene carbonate)/poly(adipic anhydride) blends. Biomaterials 21, 945–955 (2000).
Nederberg, F. et al. Organocatalytic ring opening polymerization of trimethylene carbonate. Biomacromolecules 8, 153–160 (2007).
Pratt, R. C., Nederberg, F., Waymouth, R. M. & Hedrick, J. L. Tagging alcohols with cyclic carbonate: a versatile equivalent of (meth)acrylate for ring-opening polymerization. Chem. Commun. 114–116 (2008).
Hunter, A. C. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv. Drug Deliv. Rev. 58, 1523–1531 (2006).
Deguchi, K. & Meguro, K. The determination of critical micelle concentrations of nonionic surfactants by charge-transfer solubilization of 7,7,8,8-tetracyanoquinodimethane. J. Colloid Interface Sci. 38, 596–600 (1972).
Ryjkina, E., Kuhn, H., Rehage, H., Muller, F. & Peggau, J. Molecular dynamic computer simulations of phase behavior of non-ionic surfactants. Angew. Chem. Int. Ed. 41, 983–986 (2002).
Guo, X. D., Zhang, L. J., Qian, Y. & Zhou, J. Effect of composition on the formation of poly(DL-lactide) microspheres for drug delivery systems: mesoscale simulations. Chem. Eng. J. 131, 195–201 (2007).
Srinivas, G. & Pitera, J. W. Soft patchy nanoparticles from solution-phase self-assembly of binary diblock copolymers. Nano Lett. 8, 611–618 (2008).
Barbara, E. & Murray, M. D. Vancomycin-resistant Enterococcal infections. N. Engl. J. Med. 342, 710–721 (2000).
Chang, S. et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N. Engl. J. Med. 348, 1342–1347 (2003).
Hiramatsu, K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect. Dis. 1, 147–155 (2001).
Kelly, S. L. et al. Resistance to amphotericin B associated with defective sterol Δ8→7 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS Microbiol. Lett. 122, 39–42 (1994).
Cho, S. et al. Structural insights into the bactericidal mechanism of human peptidoglycan recognition proteins. Proc. Natl Acad. Sci. USA 104, 8761–8766 (2007).
Som, A. & Tew, G. N. Influence of lipid composition on membrane activity of antimicrobial phenylene ethynylene oligomers. J. Phys. Chem. B 112, 3495–3502 (2008).
Acknowledgements
This research was supported by the National Science Foundation (NSF) Center for Polymer Interface and Macromolecular Assemblies (CPIMA; NSF-DMR-0213618) and the Institute of Bioengineering and Nanotechnology (Biomedical Research Council, Agency for Science, Technology and Research, Singapore). F.N. thanks the Swedish research council (VR) for financial support.
Author information
Authors and Affiliations
Contributions
J.L.H. and Y.Y.Y. conceived and designed the study. F.N., K.F. and C.Y. synthesized and characterized the polymers. Y.Z., J.P.K.T., K.X. and H.W. performed in vitro experiments, and S.G. carried out in vivo experiments. S.G., K.X., H.W. and L.L. contributed to in vivo data analysis. X.D.G. performed the simulation. J.L.H. and Y.Y.Y. wrote the paper, with contributions from the other authors for the Methods.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information
Supplementary information (PDF 586 kb)
Rights and permissions
About this article
Cite this article
Nederberg, F., Zhang, Y., Tan, J. et al. Biodegradable nanostructures with selective lysis of microbial membranes. Nature Chem 3, 409–414 (2011). https://doi.org/10.1038/nchem.1012
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchem.1012
This article is cited by
-
Hybrid nanoparticles of quaternary ammonium cellulose derivatives and citric acid for enhancing the antibacterial activity of polyvinyl alcohol composites
Cellulose (2023)
-
Nanotechnology: a contemporary therapeutic approach in combating infections from multidrug-resistant bacteria
Archives of Microbiology (2023)
-
A transistor-like pH-sensitive nanodetergent for selective cancer therapy
Nature Nanotechnology (2022)
-
Synthesis of alginate-based nanocomposites: a novel approach to antibacterial films
Chemical Papers (2022)
-
Engineering of hollow polymeric nanosphere-supported imidazolium-based ionic liquids with enhanced antimicrobial activities
Nano Research (2022)