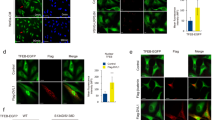
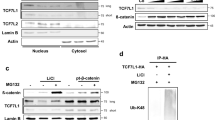
Abstract
Dishevelled (Dvl) transduces signals from the Wnt receptor, Frizzled, to downstream components, leading to the stabilization of β-catenin and subsequent activation of the transcription factor T cell factor (TCF) and/or lymphoid enchancer factor (LEF)1,2,3. However, the mechanism of Dvl action remains unclear. Here, we report that nucleoredoxin (NRX)4, a thioredoxin (TRX) family protein, interacts with Dvl. Overexpression of NRX selectively suppresses the Wnt–β-catenin pathway and ablation of NRX by RNA-interference (RNAi) results in activation of TCF, accelerated cell proliferation and enhancement of oncogenicity through cooperation with mitogen-activated extracellular signal regulated kinase kinase (MEK) or Ras. We find that cells respond to H2O2 stimulation by activating TCF. Redox-dependent activation of the Wnt–β-catenin pathway occurs independently of extracellular Wnts and is impaired by RNAi of NRX . In addition, association between Dvl and NRX is inhibited by H2O2 treatment. These data suggest a relationship between the Wnt–β-catenin pathway and redox signalling through redox-sensitive association of NRX with Dvl.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Nelson, W. J. & Nusse, R. Convergence of Wnt, β-catenin, and cadherin pathways. Science 303, 1483–1487 (2004).
Reya, T. & Clevers, H. Wnt signalling in stem cells and cancer. Nature 434, 843–850 (2005).
Moon, R. T., Kohn, A. D., De Ferrari, G. V. & Kaykas, A. WNT and β-catenin signalling: diseases and therapies. Nature Rev. Genet. 5, 691–701 (2004).
Kurooka, H. et al. Cloning and characterization of the nucleoredoxin gene that encodes a novel nuclear protein related to thioredoxin. Genomics 39, 331–339 (1997).
Laurent, T. C., Moore, E. C. & Reichard, P. Enzymatic synthesis of deoxyribonucleotides. IV. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J. Biol. Chem. 239, 3436–3444 (1964).
Chae, H. Z. et al. Cloning and sequencing of thiol-specific antioxidant from mammalian brain: alkyl hydroperoxide reductase and thiol-specific antioxidant define a large family of antioxidant enzymes. Proc. Nat. Acad. Sci. USA 91, 7017–7021 (1994).
Saitoh, M. et al. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 17, 2596–2606 (1998).
Yanagawa, S., van Leeuwen, F., Wodarz, A., Klingensmith, J. & Nusse, R. The dishevelled protein is modified by wingless signaling in Drosophila. Genes Dev. 9, 1087–1097 (1995).
Barth, A. I., Pollack, A. L., Altschuler, Y., Mostov, K.E. & Nelson, W. J. NH2-terminal deletion of β-catenin results in stable colocalization of mutant β-catenin with adenomatous polyposis coli protein and altered MDCK cell adhesion. J. Cell Biol. 136, 693–706 (1997).
Li, L. et al. Dishevelled proteins lead to two signaling pathways. Regulation of LEF-1 and c-Jun N-terminal kinase in mammalian cells. J. Biol. Chem. 274, 129–134 (1999).
Moriguchi, T. et al. Distinct domains of mouse dishevelled are responsible for the c-Jun N-terminal kinase–stress-activated protein kinase activation and the axis formation in vertebrates. J. Biol. Chem. 274, 30957–30962 (1999).
Yost, C. et al. GBP, an inhibitor of GSK-3, is implicated in Xenopus development and oncogenesis. Cell 93, 1031–1041 (1998).
Li, L. et al. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 18, 4233–4240 (1999).
Hino, S., Michiue, T., Asashima, M. & Kikuchi, A. Casein kinase Iɛ enhances the binding of Dvl-1 to Frat-1 and is essential for Wnt-3a-induced accumulation of β-catenin. J. Biol. Chem. 278, 14066–14073 (2003).
He, T. C. et al. Identification of c-MYC as a target of the APC pathway. Science 281, 1509–1512 (1998).
Tetsu, O. & McCormick, F. β-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398, 422–426 (1999).
Rimerman, R. A., Gellert-Randleman, A. & Diehl, J. A. Wnt1 and MEK1 cooperate to promote cyclin D1 accumulation and cellular transformation. J. Biol. Chem. 275, 14736–14742 (2000).
Sokol, S. Y., Klingensmith, J., Perrimon, N. & Itoh, K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development 121, 1637–1647 (1995).
Kim, C. H. et al. Repressor activity of Headless–Tcf3 is essential for vertebrate head formation. Nature 407, 913–916 (2000).
Kiecker, C. & Niehrs, C. A morphogen gradient of Wnt–β-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development 128, 4189–4201 (2001).
Michiue, T. et al. XIdax, an inhibitor of the canonical Wnt pathway, is required for anterior neural structure formation in Xenopus. Dev. Dyn. 230, 79–90 (2004).
Leyns, L., Bouwmeester, T., Kim, S. H., Piccolo, S. & De Robertis, E. M. Frzb-1 is a secreted antagonist of Wnt signaling expressed in the Spemann organizer. Cell 88, 747–756 (1997).
Wang, S., Krinks, M., Lin, K., Luyten, F. P. & Moos, M., Jr. Frzb, a secreted protein expressed in the Spemann organizer, binds and inhibits Wnt-8. Cell 88, 757–766 (1997).
Burdon, R. H. & Rice-Evans, C. Free radicals and the regulation of mammalian cell proliferation. Free Radic. Res. Commun. 6, 345–358 (1989).
Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K. & Finkel, T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 (1995).
Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 15, 247–254 (2003).
Rhee, S. G. et al. Intracellular messenger function of hydrogen peroxide and its regulation by peroxiredoxins. Curr. Opin. Cell Biol. 17, 183–189 (2005).
Miki, H., Fukuda, M., Nishida, E. & Takenawa, T. Phosphorylation of WAVE downstream of mitogen-activated protein kinase signaling. J. Biol. Chem. 274, 27605–27609 (1999).
Molenaar, M., et al. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86, 391–399 (1996).
Fujita, M. et al. Up-regulation of the ectodermal-neural cortex 1 (ENC1) gene, a downstream target of the β-catenin–T-cell factor complex, in colorectal carcinomas. Cancer Res. 61, 7722–7726 (2001).
Acknowledgements
We thank S. Ohmi and H. Fukuda for mass spectrometry analysis. We are grateful to R. J. Davis for JNK1, S. Yokoyama for RasG12V, E. Nishida for HA–MEK LA SDSE, Y. Furukawa and Y. Nakamura for wild-type TCF-4 and dominant negative TCF-4, and Y. Gotoh for β-catenin and c-Jun. We also appreciate helpful advice and support from T. Takenawa, K. Takenaka, H. Yamaguchi, T. Terabayashi, A. Yukita, K. Haraguchi, S. Aizawa and T. Chano. This study was supported in part by a Grant-in-Aid for Cancer Research from the Ministry of Education, Science, and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Figures S1, S2, S3 and Supplementary Table 1 (PDF 709 kb)
Rights and permissions
About this article
Cite this article
Funato, Y., Michiue, T., Asashima, M. et al. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt–β-catenin signalling through Dishevelled. Nat Cell Biol 8, 501–508 (2006). https://doi.org/10.1038/ncb1405
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb1405
This article is cited by
-
Monoamine oxidase A-dependent ROS formation modulates human cardiomyocyte differentiation through AKT and WNT activation
Basic Research in Cardiology (2023)
-
Kidney and epigenetic mechanisms of salt-sensitive hypertension
Nature Reviews Nephrology (2021)
-
Importance of the renal ion channel TRPM6 in the circadian secretion of renin to raise blood pressure
Nature Communications (2021)
-
Non-thermal atmospheric pressure plasma activates Wnt/β-catenin signaling in dermal papilla cells
Scientific Reports (2021)
-
Importance of wnt-catenin signaling in hypertensive kidney diseases
Hypertension Research (2021)