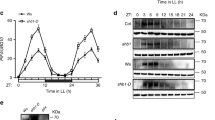
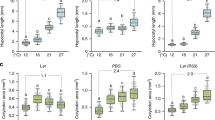
Abstract
The transition to flowering in plants is regulated by environmental factors such as temperature and light1. Plants grown under dense canopies or at high density perceive a decrease in the ratio of red to far-red incoming light. This change in light quality serves as a warning of competition, triggering a series of responses known collectively as the ‘shade-avoidance syndrome’. During shade avoidance, stems elongate at the expense of leaf expansion, and flowering is accelerated2,3. Of the five phytochromes—a family of red/far-red light photoreceptors—in Arabidopsis, phytochrome B (phyB) has the most significant role in shade-avoidance responses4,5, but the mechanisms by which phyB regulates flowering in response to altered ratios of red to far-red light are largely unknown. Here we identify PFT1 (PHYTOCHROME AND FLOWERING TIME 1), a nuclear protein that acts in a phyB pathway and induces flowering in response to suboptimal light conditions. PFT1 functions downstream of phyB to regulate the expression of FLOWERING LOCUS T (FT), providing evidence for the existence of a light-quality pathway that regulates flowering time in plants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Levy, Y. Y. & Dean, C. Control of flowering time. Curr. Opin. Plant Biol. 1, 49–54 (1998)
Ballare, C. L. Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plant Sci. 4, 201 (1999)
Halliday, K. J., Koornneef, M. & Whitelam, G. C. Phytochrome B and at least one other phytochrome mediate the accelerated flowering response of Arabidopsis thaliana L. to low red/far-red ratio. Plant Physiol. 104, 1311–1315 (1994)
Aukerman, M. J. et al. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell 9, 1317–1326 (1997)
Devlin, P. F., Patel, S. R. & Whitelam, G. C. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 10, 1479–1487 (1998)
Lin, C. Photoreceptors and regulation of flowering time. Plant Physiol. 123, 39–50 (2000)
Quail, P. H. Phytochrome photosensory signalling networks. Nature. Rev. Mol. Cell Biol. 3, 85–93 (2002)
Yanovsky, M. J. & Kay, S. A. Molecular basis of seasonal time measurement in Arabidopsis. Nature 419, 308–312 (2002)
Simpson, G. G. & Dean, C. Arabidopsis, the Rosetta stone of flowering time? Science 296, 285–289 (2002)
Cerdan, P. D. et al. Regulation of phytochrome B signaling by phytochrome A and FHY1 in Arabidopsis thaliana. Plant J. 18, 499–507 (1999)
Hennig, L., Poppe, C., Sweere, U., Martin, A. & Schafer, E. Negative interference of endogenous phytochrome B with phytochrome A function in Arabidopsis. Plant Physiol. 125, 1036–1044 (2001)
Mockler, T. et al. Regulation of photoperiodic flowering by Arabidopsis photoreceptors. Proc. Natl Acad. Sci. USA 100, 2140–2145 (2003)
Ponting, C. P., Schultz, J., Milpetz, F. & Bork, P. SMART: identification and annotation of domains from signalling and extracellular protein sequences. Nucleic Acids Res. 27, 229–232 (1999)
Ponting, C. P., Aravind, L., Schultz, J., Bork, P. & Koonin, E. V. Eukaryotic signalling domain homologues in archaea and bacteria. Ancient ancestry and horizontal gene transfer. J. Mol. Biol. 289, 729–745 (1999)
Escher, D., Bodmer-Glavas, M., Barberis, A. & Schaffner, W. Conservation of glutamine-rich transactivation function between yeast and humans. Mol. Cell. Biol. 20, 2774–2782 (2000)
Hinshelwood, J. & Perkins, S. J. Metal-dependent conformational changes in a recombinant vWF-A domain from human factor B: a solution study by circular dichroism, fourier transform infrared and 1H NMR spectroscopy. J. Mol. Biol. 298, 135–147 (2000)
Levy, Y. Y., Mesnage, S., Mylne, J. S., Gendall, A. R. & Dean, C. Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science 297, 243–246 (2002)
Kardailsky, I. et al. Activation tagging of the floral inducer FT. Science 286, 1962–1965 (1999)
Kobayashi, Y., Kaya, H., Goto, K., Iwabuchi, M. & Araki, T. A pair of related genes with antagonistic roles in mediating flowering signals. Science 286, 1960–1962 (1999)
Suarez-Lopez, P. et al. CONSTANS mediates between the circadian clock and the control of flowering in Arabidopsis. Nature 410, 1116–1120 (2001)
Blazquez, M. A. & Weigel, D. Independent regulation of flowering by phytochrome B and gibberellins in Arabidopsis. Plant Physiol. 120, 1025–1032 (1999)
Putterill, J., Robson, F., Lee, K., Simon, R. & Coupland, G. The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80, 847–857 (1995)
Samach, A. et al. Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288, 1613–1616 (2000)
Hepworth, S. R., Valverde, F., Ravenscroft, D., Mouradov, A. & Coupland, G. Antagonistic regulation of flowering-time gene SOC1 by CONSTANS and FLC via separate promoter motifs. EMBO J. 21, 4327–4337 (2002)
Lee, H. et al. The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev. 14, 2366–2376 (2000)
Koornneef, M., Hanhart, C., Van Loenen-Martinet, P. & Blankestijn-de Vries, H. The effect of daylength on the transition to flowering in phytochrome-deficient, late-flowering and double mutants of Arabidopsis thaliana. Physiol. Plant 95, 260–266 (1995)
Blazquez, M. A., Trenor, M. & Weigel, D. Independent control of gibberellin biosynthesis and flowering time by the circadian clock in Arabidopsis. Plant Physiol. 130, 1770–1775 (2002)
Acknowledgements
We thank the Kasuza Research Institute and the Arabidopsis Stock Center for providing EST clone APZL03h11R and BAC F2J7, respectively; D. Weigel for providing FT- and CO-overexpressing lines, and for useful advice; P. Wigge, S. Mora-Garcia, D. Weigel, J. Nemhauser, M. Yanovsky and J. Casal for comments on the manuscript; L. Barden for figure preparation; and B. Smoot, N. Ga and M. Ledgerwood for technical assistance. This work was supported by a grant from the National Institutes of Health to J.C. (GM52413) and by the Howard Hughes Medical Institute. P.C. was a fellow of the PEW Latin American Fellows Program and Fundación Antorchas.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Cerdán, P., Chory, J. Regulation of flowering time by light quality. Nature 423, 881–885 (2003). https://doi.org/10.1038/nature01636
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature01636
This article is cited by
-
Effects of LEDs Light Spectra on the Growth, Yield, and Quality of Winter Wheat (Triticum aestivum L.) Cultured in Plant Factory
Journal of Plant Growth Regulation (2023)
-
Comparative analysis of buds transcriptome and identification of two florigen gene AkFTs in Amorphophallus konjac
Scientific Reports (2022)
-
Multi-locus genome-wide association studies reveal novel alleles for flowering time under vernalisation and extended photoperiod in a barley MAGIC population
Theoretical and Applied Genetics (2022)
-
Environment-mediated mutagenetic interference on genetic stabilization and circadian rhythm in plants
Cellular and Molecular Life Sciences (2022)
-
Prediction of strawberry fruit yield based on cultivar-specific growth models in the tunnel-type greenhouse
Horticulture, Environment, and Biotechnology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.