Abstract
In the past decade, colloidal solutions have been assumed to be very efficient templates for controlling particle size and shape. A large number of groups have used reverse micelles to control the size of spherical nanoparticles. This makes it possible to determine the various parameters involved in such processes, and demonstrates that nanoparticles can be considered to be efficient nanoreactors. However, some discrepancies arise. There are few reports concerning the control of particle shape, and it is still rather difficult to determine the key parameters, such as the adsorption of salts and other molecules, and the synthesis procedure. Here, we discuss these controls of the size and shape of inorganic nanomaterials.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Heath, J.R. Nanoscale materials. Acc. Chem. Res. 32, 388–388 (1999).
Vollath, D., Szabo, D.V., Taylor, R.D. & Willis, J.O. Synthesis and magnetic properties of nanostructured maghemite. J. Mat. Res. 12, 2175–2182 (1997).
Perez, A. et al. Nanostructured materials from clusters: synthesis and properties. Mater. Trans. 42, 1460–1470 (2001).
Murray, C.B., Norris, D.J. & Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E= sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 115, 8706–8715 (1993).
Pileni, M.P. Reverse micelles: a microreactor. J. Phys. Chem. 97, 9661–9668 (1993).
Pileni, M.P. Nanosized particles made in colloidal assemblies. Langmuir 13, 3266–3276 (1997).
Pileni, M.P. Mesostructured fluids in oil rich region: Structural and templating approaches. Langmuir 17, 7476–7486 (2001).
Cepak, V.M. & Martin, C.R. Preparation and stability of template synthesized metal nanorod sols in organic solvents. J. Phys. Chem. 102, 9985–9990 (1998).
Molares, M.E.T. et al. Single crystalline copper nanowires produced by electrochemical deposition in polymeric ion track membrane. Adv. Mater. 13, 62–65 (2001).
Namatsu, H., Kurihara, K., Nagase, M. & Makino, T. Fabrication of 2-nm wide silicon quantum wires through a combination of a partially shifted resist pattern and orientation dependent etching. Appl. Phys. Lett. 70, 619–621 (1997).
Wang, J., Thomson, D.A., Robinson, B.J. & Simmons, J.G. Molecular beam epitaxial growth of InGaAs/InGaAsP quantum wires on V-grooved InP substrates with (111) sidewalls. J. Cryst. Growth 175, 793–798 (1997).
Nepojko, S.A., Levlev, D.N., Schulze, W., Urban, J. & Ertl, G. Growth of rodlike silver nanoparticles by vapor deposition of small clusters. Chem. Phys. Chem. 140–142 (2000).
Yu, Y.Y., Chang, S.S., Lee C.L. & Wang, C.R. Gold nanorods: electrochemical synthesis and optical properties. J. Phys. Chem. B 101, 6661–6664 (1997).
Huang, L. et al. Nanowires arrays electrodeposited from liquid crystalline phases. Adv. Mater. 14, 61–64 (2002).
Wang, Z.L., Gao, R.P., Nikoobakht, B. & El Sayed, M.A. Surface reconstruction of unstable [110] surface in gold nanorods. J. Phys. Chem. B 104, 5417–5420 (2000).
Evans, D.F., Mitchell D.J. & Ninham, B.W. Oil, water and surfactant: properties and conjectured structure of simple microemulsions. J. Phys. Chem. 90, 2817–2825, (1986).
Pileni, M.P. (ed.) Reverse micelles (Elsevier, Amsterdam, 1989).
Pileni, M.P., Zemb, T. & Petit, C. Solubilization by reverse micelles: Solute localization and structure perturbation. Chem. Phys. Lett. 118, 414–420 (1985).
The Language of Shape: The Role of Curvature in Condensed Matter: Physics, Chemistry and Biology (eds Hyde, S. et al.) (Elsevier, Oxford, 1997).
André, P. et al. Supra-aggregation: Microphase formation in complex fluids. Adv. Mater. 12, 119–123 (2000).
Petit, C. & Pileni, M.P. Synthesis of cadmium sulfide in situ in reverse micelles and hydrocarbon gels. J. Phys. Chem. 92, 2282–2286 (1988).
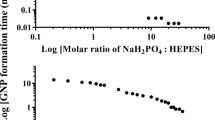
Ingert, D. & Pileni, M.P. Limitation in producing nanomaterials by using reverse micelles as nanoreactors. Adv. Funct. Mater. 11, 136–139 (2001).
Weller, H. Colloidal semiconductor q particles: Chemistry in the transition region between solid state and molecules. Angew. Chem. Int. Edn Engl. 32, 41–53 (1993).
Courty, A., Lisiecki, I. & Pileni, M.P. Vibration of self-organized silver nanocrystals. J. Chem. Phys. 116, 8074–8078 (2002).
Pileni, M.P. II-VI Semiconductors made by solf chemistry: Syntheses and optical properties. Catal. Today 58, 151–166 (2000).
Agnoli, F. Zhou, W.L. & O'Connor, C.J. Synthesis of cubic antiferromagnetic KMnF3 Nanoparticles using reverse micelles and their assembly. Adv. Mater. 13, 1697–1699 (2001).
Pinna, N., Weiss, K., Urban, J. & Pileni, M.P. Triangular CdS nanocrystals: Structure and optical Properties. Adv. Mater. 13, 261–264 (2001).
Hopwood, J.D. & Mann, S. Synthesis of barium nanoparticles and nanofilament in reverse micelles and microemulsions. Chem. Mater. 9, 1819–1828 (1997).
Li, M. Schnablegger, H. & Mann, S. Coupled synthesis and self-assembly of nanoparticles to give structures with controlled organization. Nature 402, 393–396 (1999).
Rees, G.D., Evans-Gowing, R., Hammond, S.J. & Robinson, B.H. Formation and morphology of calcium sulfate nanoparticles and nanowires in water in oil microemulsions. Langmuir 15, 1993–2002, (1999).
Qi, L., Ma, J., Cheng, H. & Zhao, Z. Reverse micelles based formation of BaCO3 Nanowires. J. Phys. Chem. B 101, 3460–3463 (1997).
Filankembo, A. et al. Mesostructured fluids: supra aggregates made of interdigitated reverse micelles. Colloids Surf. A 174, 221–232 (2000).
Lisiecki, I. et al. Structural investigations of copper nano-rods by HRTEM. Phys. Rev. B 61, 4968–4974 (2000).
Simmons, B.A. et al. Morphology of CdS nanocrystals synthesized in mixed surfactant systems. Nanolett. 2, 263–268 (2002).
Scartazzini, R. & Luisi, P. Organogels from lecithins. J. Phys. Chem. 92, 829–933, (1988).
Filankembo, A. & Pileni, M.P. Is the template of self-colloidal assemblies the only factor which controls nanocrystals shapes? J. Phys. Chem. B 104, 5865–5868 (2000).
Tanori, J. & Pileni, M.P. Change in the shape of copper nanoparticles in ordered phases. Adv. Mater. 7, 862–864 (1995).
Chen, C.C. & Lin, J.J. Controlled growth of cubic cadmium sulfide nanoparticles using patterned self-assembled monolayers as template. Adv. Mater. 13, 136–139 (2001).
Henglein, A. & Giersig. M. Reduction of Pt(II) by H2: Effects of citrate and NaOH and chemical mechanism. J. Phys. Chem. 104, 6767–6772 (2000).
Ahmadi, T.S., Wang, Z.L., Heinglein, A. & El Sayed, M.A. Cubic colloidal platinium nanoparticles. Chem. Mater. 8, 1161–1163 (1996).
Esumi, K., Matsuhira, K. & Torigoe, K. Preparation of rodlike gold particles by UV irradiation using cationic micelles as template. Langmuir 11, 3285–3287 (1995).
Maillard, M., Giorgio, S. & Pileni, M.P. Silver nanodisks. Adv. Mater. 14, 1084–1086, (2002).
Maillard, M., Giorgio, S. & Pileni, M.P. Tuning the size of silver nanodisks with similar aspect ratio: synthesis and optical properties. J. Phys. Chem. B (in the press).
Li, Y., Ding, Y. & Wang, Z. Novel route to ZnTe semiconductor nanorods. Adv. Mater. 11, 847–850 (1999).
Li, Y. et al. Solvothermal elemental direct reaction to CdE (E= S, Se, Te) semiconductor nanorod. Inorg. Chem. 38, 1382–1387 (1999).
Wang, S. & Yang, S. Preparation and characterization of oriented PbS crystalline nanorods in polymer films. Langmuir 16, 389–397 (2000).
Manna, L., Scher, E.C. & Alivisatos, A.P. Synthesis of soluble and processable rod-, arrow-, teardrop-, and tetrapod-shaped CdSe nanocrystals. J. Am. Chem. Soc. 122, 12700–12706 (2000).
Puntes, V.F., Kristhnan, K.M. & Alivisatos, A.P. Colloidal nanocrystal shape and size control: the case of cobalt. Science 291, 2115–2118 (2001).
Park, S.J. et al. Synthesis and magnetic studies of uniform iron nanorods and nanospheres. J. Am. Chem. Soc. 122, 8581–8582 (2000).
Jana, N.R. Gearheart, L. & Murphy, C. Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio. Chem. Commun. 617–618 (2001).
Jana, N.R. Gearheart, L. & Murphy, C.J. Wet chemical synthesis of high aspect ratio cylindrical gold nanorods. J. Phys. Chem. B 105, 4065–4067 (2001).
Boistelle, R. in Industrial Crystallization (ed. Mullin, J.W.) 203–214 (Plenum, New York 1976).
Pileni, M.P. Ferrite magnetic fluids: A new fabrication method and magnetic properties of nanocrystals differing by their size and composition. Adv. Funct. Mater. 11, 323–333 (2001).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Pileni, MP. The role of soft colloidal templates in controlling the size and shape of inorganic nanocrystals. Nature Mater 2, 145–150 (2003). https://doi.org/10.1038/nmat817
Issue Date:
DOI: https://doi.org/10.1038/nmat817
This article is cited by
-
Magnetic Iron Oxide Nanoparticles as a Tool for the Advancement of Biomedical and Environmental Application: A Review
Biomedical Materials & Devices (2024)
-
Caterpillar-shaped hierarchical ZSM-5 resulted from the self-assembly of regularly primary nano-sized zeolite crystals
Journal of Porous Materials (2023)
-
Selective and Ultrasensitive Spectroscopic Detection of Mercuric Ion in Aqueous Systems Using Embonic Acid Functionalized Silver Nanoparticle
Journal of Cluster Science (2023)
-
The characterization of hexagonal lyotropic liquid crystal nanostructure: effects of polymer tail length
Colloid and Polymer Science (2022)
-
Enhancement of magnetic and dielectrics performance of BaFe2O4 nanoparticles influenced by tri-sodium citrate as surfactant
Journal of Materials Science: Materials in Electronics (2022)