Abstract
The earliest event so far known that occurs in the brain affected with Alzheimer's disease (AD) is the deposition and fibril formation of amyloid β–protein (Aβ). Aβ is cleaved from a glycosylated membrane protein, called β–amyloid protein precursor, and normally secreted into the extracellular space. Here we report on the presence of membrane–bound Aβ that tightly binds CM1 ganglioside. This suggests that this novel Aβ species, rather than secreted Aβ, may act as a ‘seed’ for amyloid and further that intracellular abnormalities in the membrane recycling already exist at the stage of amyloidogenesis
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Mullan, M. & Crawford, F. Genetic and molecular advances in Alzheimer's disease. Trends Neurosd. 16, 398–403 (1993).
Selkoe, D.J. Cell biology of the amyloid β-protein precursor and the mechanism of Alzheimer's disease. Annu. Rev. CellBiol. 10, 373–403 (1994).
Kang, J. et al. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature 325, 733–736 (1987).
Mori, H., Takio, K., Ogawara, M. & Selkoe, D.J. Mass spectrometry of purified amyloid β protein in Alzheimer's disease. J. biol. Chem. 267, 17082–17086 (1992).
Roher, A.E. et al. Structural alteration in the peptide backbone of β-amyloid core protein may account for its deposition and stability in Alzheimer's disease. J. biol. Chem. 268, 3072–3083 (1993).
Yamaguchi, H., Nakazato, Y., Hirai, S., Shoji, M. & Harigaya, Y. Electron micrograph of diffuse plaques: Initial stage of senile plaque formation in the Alzheimer brain. Am. J. Pathol 135, 593–597 (1989).
Davies, C.A. & Mann, D.M.A. Is the “preamyloid” of diffuse plaques in Alzheimer's disease really nonfibrillar? Am. J. Pathol. 143, 1594–1605 (1993).
Yamaguchi, H. et al. A variety of cerebral amyloid deposits in the brains of the Alzheimer-type dementia demonstrated by β protein immunostaining. Ada Neuropathol. 76, 541–549 (1988).
Yamaguchi, H., Nakazato, Y., Hirai, S. & Shoji, M. Immunoelectron microscopic localization of amyloid β protein in the diffuse plaques of the Alzheimer-type dementia. Brain Res. 508, 320–324 (1990).
Yamazaki, T. et al. Ultrastructural characterization of cerebellar diffuse plaques in Alzheimer's disease. J. Neuropathol. exp. Neurol. 51, 281–286 (1992).
Suzuki, N. et al. An increased percentage of long amyloid β protein secreted by familial amyloid β protein precursor (βAPP 717) mutants. Science 264, 1336–1340 (1994).
Iwatsubo, T. et al. Visualization of Aβ42 (43) and Aβ40 in senile plaques with end-specific Aβ monoclonals: Evidence that an initially deposited species is Ap42 (43). Neuron 13, 45–53 (1994).
Mann, D.M.A. Cerebral amyloidosis, ageing and Alzheimer's disease: A contribution from studies on Down's syndrome. Neurobiol. Aging 10, 397–399 (1989).
Masters, C.L. et al. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Prot. natn. Acad. Sci. U.S.A. 82, 4245–4249 (1985).
Suzuki, N. et al. High tissue content of soluble β1-40 is linked to cerebral amyloid angiopathy. Am. J. Pathol. 145, 452–460 (1994).
Kim, K.S. et al. Production and characterization of monoclonal antibodies reactive to synthetic cerebrovascular amyloid peptide. Neurosd. Res. Commun. 2, 121–130 (1988).
Haass, C. et al. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature 359, 322–325 (1992).
Seubert, P. et al. Isolation and quantitation of soluble Alzheimer's β-peptide from biological fluids. Nature 359, 325–327 (1992).
Shoji, M. et al. Production of the Alzheimer amyloid β protein by normal proteolytic processing. Science 258, 126–129 (1992).
Pike, C.J., Walencewicz, A.J., Glabe, C.G. & Cotman, C.W. In vitro aging of β-amyloid protein causes peptide aggregation and neurotoxicity. Brain Res. 563, 311–314 (1991).
Braak, H. & Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82, 239–259 (1991).
Pan, B.-T. & Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 33, 967–977 (1983).
Hansson, H.A., Holmgren, J. & Svennerholm, L. Ultrastructural localization of cell membrane GM1 ganglioside by cholera toxin. Proc. natn. Acad. Sci. U.S.A. 74, 3782–3786 (1977).
Parton, R.G. Ultrastructural localization of gangliosides: GM1 is concentrated in caveolae. J. Histochem. Cytochem. 42, 155–166 (1994).
Dupree, P. et al. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 12, 1597–1605 (1993).
Sherrington, R. et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature 375, 754–760 (1995).
L'Hernault, S.W.L. & Arduengo, P.M. Mutation of a putative sperm membrane protein in Caenorhabditis elegans prevents sperm differentiation but not its associated meiotic divisions. J. Cell Biol. 119, 55–69 (1992).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yanagisawa, K., Odaka, A., Suzuki, N. et al. GM1 ganglioside–bound amyloid β–protein (Aβ): A possible form of preamyloid in Alzheimer's disease. Nat Med 1, 1062–1066 (1995). https://doi.org/10.1038/nm1095-1062
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nm1095-1062
This article is cited by
-
Recently developed glycosphingolipid probes and their dynamic behavior in cell plasma membranes as revealed by single-molecule imaging
Glycoconjugate Journal (2023)
-
Glucosylceramide synthase inhibition reduces ganglioside GM3 accumulation, alleviates amyloid neuropathology, and stabilizes remote contextual memory in a mouse model of Alzheimer’s disease
Alzheimer's Research & Therapy (2022)
-
Immuno-digital invasive cleavage assay for analyzing Alzheimer’s amyloid ß-bound extracellular vesicles
Alzheimer's Research & Therapy (2022)
-
Structure and function of glycosphingolipids on small extracellular vesicles
Glycoconjugate Journal (2022)
-
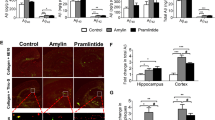
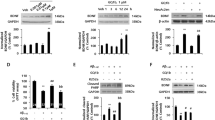
Amylin and pramlintide modulate γ-secretase level and APP processing in lipid rafts
Scientific Reports (2020)