Abstract
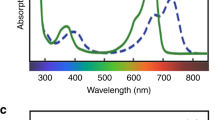
For plants, the sensing of light in the environment is as important as vision is for animals. Fluctuations in light can be crucial to competition and survival. One way plants sense light is through the phytochromes, a small family of diverse photochromic protein photoreceptors whose origins have been traced to the photosynthetic prokaryotes. During their evolution, the phytochromes have acquired sophisticated mechanisms to monitor light. Recent advances in understanding the molecular mechanisms of phytochromes and their significance to evolutionary biology make possible an interim synthesis of this rapidly advancing branch of photobiology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Neff, M. M., Fankhauser, C. & Chory, J. Light: an indicator of time and place. Genes Dev. 14, 257–271 ( 2000).
Ahmad, M. & Cashmore, A. R Seeing blue: The discovery of cryptochrome. Plant Mol. Biol. 30, 851– 861 (1996).
Christie, J. M. et al. Arabidopsis NPH1: A flavoprotein with the properties of a photoreceptor for phototropism. Science 282, 1698–1701 (1998).
Smith, H. Light quality, photoperception and plant strategy. Annu. Rev. Plant Physiol. 33, 481–518 ( 1982).
Ballare, C. L., Scope, A. L., Sanchez, R. A., Casal, J. J. & Ghersa, C. M. Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Env. 10, 551–557 (1987).
Gilbert, I. R., Seavers, G. P., Jarvis, P. G. & Smith, H. Photomorphogenesis and canopy dynamics. Phytochrome-mediated proximity perception accounts for the growth dynamics of canopies of Populus trichocarpa X deltoides ‘Beaupré’. Plant Cell Env. 18, 475–497 (1995).
Smith, H. Physiological and ecological function within the phytochrome family. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 289– 315 (1995).
Clack, T., Mathews, S. & Sharrock, R. A. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol. Biol. 25, 413 –427 (1994).
Mathews, S. & Sharrock, R. A. The phytochrome gene family in grasses (Poaceae): A phylogeny and evidence that grasses have a subset of the loci found in dicot angiosperms. Mol. Biol. Evol. 13, 1141–1150 (1996).
Mathews, S. & Sharrock, R. A. Phytochrome gene diversity. Plant Cell Env. 20, 666– 671 (1997).
Alba, R., Kelmenson, P. M., Cordonnier-Pratt, M. M. & Pratt, L. H. The phytochrome gene family in tomato and the rapid differential evolution of this family in angiosperms. Mol. Biol. Evol. 17, 362–373 (2000).
Kehoe, D. M. & Grossman, A. R. Similarity of a chromatic adaptation sensor to phytochrome and ethylene receptors. Science 273, 1409–1412 (1996).
Hughes, J. et al. A prokaryotic phytochrome. Nature 386 , 663 (1997).
Yeh, K. C., Wu, S. H., Murphy, J. T. & Lagarias, J. C. A cyanobacterial phytochrome two-component light sensory system. Science 277, 1505–1508 (1997).
Jiang, Z. Y. et al. Bacterial photoreceptor with similarity to photoactive yellow protein and plant phytochromes. Science 285, 406–409 (1999).
Davis, S. J., Vener, A. V. & Vierstra, R. D. Bacteriophytochromes: phytochrome-like photoreceptors from nonphotosynthetic eubacteria. Science 286, 2517–2520 (1999).
Esch, H., Hartmann, E., Cove, D., Wada, M. & Lamparter, T. Phytochrome-controlled phototropism of protonemata of the moss Ceratodon purpureus: physiology of the wild type and class 2 ptr-mutants. Planta 209, 290– 298 (1999).
Nozue, K. et al. A phytochrome from the fern Adiantum with features of the putative photoreceptor NPH1. Proc. Natl Acad. Sci. USA 95, 15826–15830 (1998).
Thümmler, F., Dufner, M., Kreisl, P. & Dittrich, P. Molecular cloning of a novel phytochrome gene of the moss Ceratodon-purpureus which encodes a putative light-regulated protein-kinase. Plant Mol. Biol. 20, 1003–1017 (1992).
Whitelam, G. C. & Devlin, P. F. Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Env. 20, 752–75 ( 1997).
Smith, H., Xu, Y. & Quail, P. H. Antagonistic but complementary actions of phytochromes A and B allow optimum seedling de-etiolation. Plant Physiol. 114, 637–641 (1997).
Tobin, E. M. & Kehoe, D. M Phytochrome regulated gene expression. Semin. Cell Biology 5, 335– 346 (1994).
Kuno, N., Muramatsu, T., Hamazato, F. & Furuya, M. identification by large-scale screening of phytochrome-regulated genes in etiolated seedlings of Arabidopsis thaliana using a fluorescent differential display technique. Plant Physiol. 122, 15 –22 (2000).
Shacklock, P. S., Read, N. D. & Trewavas, A. J. Cytosolic free calcium mediates red light-induced photomorphogenesis. Nature 358, 753– 755 (1992).
Smith, H., Jackson, G. M. Rapid phytochrome regulation of wheat seedling extension. Plant Physiol. 84, 1059–1062 (1987).
Parks, B. M. & Spalding, E. P. Sequential and coordinated action of phytochromes A and B. Proc. Natl Acad. Sci. USA 96, 14142–14146 (1999).
Wada, M., Grolig, F. & Haupt, W. Light-oriented chloroplast positioning—contribution to progress in photobiology. J. Photochem. Photobiol. B 17, 3–25 (1993).
Quail, P. H. et al. Phytochromes-photosensory perception and signal-transduction. Science 268, 675–680 (1995).
Cashmore, A. R. Higher-plant phytochrome: “I used to date histidine, but now I prefer serine”. Proc. Natl Acad. Sci. USA 95, 13358–13360 (1998).
Schneider-Poetsch, H. A. W. Signal transduction by phytochrome- phytochromes have a module related to the transmitter modules of bacterial sensor proteins. Photochem. Photobiol. 56, 839–846 ( 1992).
Yeh, K. C. & Lagarias, J. C. Eukaryotic phytochromes: Light-regulated serine/threonine protein kinases with histidine kinase ancestry. Proc. Natl Acad. Sci. USA 95, 13976– 13981 (1998).
Fankhauser, C. et al. PKS1, a substrate phosphorylated by phytochrome that modulates light signaling in Arabidopsis. Science 284, 1539–1541 (1999).
Ahmad, M., Jarillo, J. A., Smirnova, O. & Cashmore, A. R. The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol. Cell, 1, 939– 948 (1998).
Choi, G. et al. Phytochrome signalling is mediated through nucleoside diphosphate kinase 2. Nature 401, 610– 613 (1999).
Ni, M., Tepperman, J. M. & Quail, P. H. PIF3, a phytochrome-interacting factor necessary for normal photoinduced signal transduction, is a novel basic helix-loop-helix protein. Cell 95, 657–667 (1998).
Ni, M., Tepperman, J. M. & Quail, P. H. Binding of phytochrome B to its nuclear signalling partner PIF3 is reversibly induced by light. Nature 400, 781–784 (1999).
Halliday, K. J., Hudson, M., Ni, M., Qin, M. M. & Quail, P. H. poc1: An Arabidopsis mutant perturbed in phytochrome signaling because of a T DNA insertion in the promoter of PIF3, a gene encoding a phytochrome-interacting bHLH protein. Proc. Natl Acad. Sci. USA 96, 5832–5837 ( 1999).
Martinez-Garcia, J. F., Huq, E. & Quail, P. H. Direct targeting of light signals to a promoter element-bound transcription factor. Science 288, 859– 863 (2000).
Terzaghi, W. B. & Cashmore, A. R. Light-regulated transcription. Annu. Rev. Plant Physiol. Plant Mol. Biol. 46, 445–474 (1995).
Nagy, F. & Schäfer, E. Nuclear and cytosolic events of light-induced, phytochrome-regulated signaling in higher plants. EMBO J. 19: 157-163 (2000).
Kircher, S. et al. Light quality-dependent nuclear import of the plant photoreceptors phytochromes A and B. Plant Cell 11, 1445 –1456 (1999).
Yamaguchi, R., Nakamura, M., Mochzuki, N., Kay, S. A. & Nagatani, A. Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis . J. Cell Biol. 145, 437– 445 (1999).
Bognar, L. K. et al. The circadian clock controls the expression pattern of the circadian input photoreceptor, phytochrome B. Proc. Natl Acad. Sci. USA 96, 14652–14657 (1999).
Bradshaw, A. D. Evolutionary significance of phenotypic plasticity in plants. Annu. Rev. Genet. 13, 115–155 (1965).
Schlichting, C. D. The evolution of phenotypic plasticity in plants. Annu. Rev. Ecol. Systematics 17, 667–693 (1986).
Sultan, S. E. Evolutionary implications of phenotypic plasticity in plants. Evol. Biol. 21, 127–178 (1987).
Via, S. et al. Adaptive phenotypic plasticity: consensus and controversy. Trends Ecol. Evol. 10, 212–216 (1995).
Van Tienderen, P. H. & Koelewijn, H. P. Selection on reaction norms, genetic correlations and constraints. Genet. Res. 64, 115–125 ( 1994).
Callahan, H. S., Pigliucci, M. & Schlichting, C. D. Developmental phenotypic plasticity: where ecology and evolution meet molecular biology. BioEssays 19, 519–525 (1997).
Schmitt, J., Dudley, S. A. & Pigliucci, M. Manipulative approaches to testing adaptive plasticity: phytochrome-mediated shade-avoidance responses in plants. Am. Nat. 154, S43–S54 ( 1999).
Ballare, C. L. & Scopel, A. L. Phytochrome signalling in plant canopies: testing its population-level implications with photoreceptor mutants of Arabidopsis. Funct. Ecol. 11, 441–450 (1997).
Pigliucci, M. & Schmitt, J. Genes affecting phenotypic plasticity in Arabidopsis: pleiotropic effects and reproductive fitness of photomorphogenic mutants. J. Evol. Biol. 12, 551-562 (1999).
Schmitt, J., McCormac, A. C., Smith, H. A test of the adaptive plasticity hypothesis using transgenic and mutant plants disabled in phytochrome-mediated elongation responses to neighbors. Am. Nat. 146, 937– 953 (1995).
Smith, H. Signal perception, differential expression within multigene families and the molecular basis of phenotypic plasticity. Plant Cell Env. 13, 585–594 (1990).
McSteen, P. & Hake, S. Genetic control of plant development. Curr. Op. Biotechnol. 9, 189– 195 (1998).
Purugannan, M. D. The molecular genetics of regulatory genes. Mol. Ecol. 9, 1451–1462 (2000)
Meyerowitz, E. M. Plants, animals and the logic of development. Trends Biochem. Sci. 24, M65–M68 ( 1999)
Ting, C. T., Tsaur, S. C., Wu, M. L. & Wu, C. I. A rapidly evolving homeobox at the site of a hybrid sterility gene. Science 282, 1501–1504 (1998).
Purugannan, M. D., Rounsley, S. D., Schmidt, R. J. & Yanofsky, M. F. Molecular evolution of flower development: Diversification of the plant MADS-box regulatory gene family. Genetics 140, 354 –356 (1995).
Mitchell-Olds, T. The molecular-basis of quantitative genetic-variation in natural-populations. Trends Ecol. Evol. 10, 324– 328 (1995).
Alonso-Blanco, C. & Koornneef, M. Naturally occurring variation in Arabidopsis: an underexploited resource for plant genetics. Trends Plant Sci. 5, 22– 29 (2000).
Alonso-Blanco, C., Blankestijn-de Vries, H., Hanhart, C. J. & Koornneef, M. Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 96, 4710–4717 ( 1999).
Alonso-Blanco, C., El-Assal, S. E., Coupland, G. & Koornneef, M. Analysis of natural allelic variation at flowering time loci in the Landsberg erecta and Cape Verde Isles ecotypes of Arabidopsis thaliana. Genetics 149, 749–764 ( 1998).
Swarup, K. et al. Natural allelic variation identifies new genes in the Arabidopsis circadian system. Plant J. 20, 67–77 (1999).
Robson, P. R. H., McCormac, A. C., Irvine, A. S. & Smith, H. Genetic engineering of harvest index in tobacco through overexpression of a phytochrome gene. Nature Biotechnol. 14, 995–998 (1996).
Olsen, J. E. et al. Ectopic expression of oat phytochrome A in hybrid aspen changes critical daylength for growth and prevents cold acclimatization. Plant J. 12, 1339–1350 ( 1997).
Donoghue, M. J. & Mathews, S. Duplicate genes and the root of angiosperms, with an example using phytochrome sequences. Molecular Phylogenet. Evol. 9, 489– 500 (1998).
Mathews, S. & Donoghue, M. J. The root of angiosperm phylogeny inferred from duplicate phytochrome genes. Science 286, 947–950 (1999).
Quail, P. H. An emerging molecular map of the phytochrome. Plant Cell Environment 20, 657–665 ( 1997).
Whitelam, G. C. & Devlin, P. F. Roles of different phytochromes in Arabidopsis photomorphogenesis. Plant Cell Env. 20, 752–758 ( 1997).
Robson, P. R. H. & Smith, H. Fundamental and biotechnological applications of the phytochromes. Plant Cell Env. 20, 831–839 ( 1997).
Acknowledgements
I thank the following for information and comment: J. Chory, M. Furuya, J. C. Lagarias, P. H. Quail, J. Schmitt, P.-S. Song. The author's research on shade avoidance was supported by the UK Natural Environment Research Council, the UK Biotechnology and Biological Sciences Research Council and the Council of the European Commission. The literature survey for this article was completed on 31 March 2000.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smith, H. Phytochromes and light signal perception by plants—an emerging synthesis. Nature 407, 585–591 (2000). https://doi.org/10.1038/35036500
Issue Date:
DOI: https://doi.org/10.1038/35036500
This article is cited by
-
The mechanism of low blue light-induced leaf senescence mediated by GmCRY1s in soybean
Nature Communications (2024)
-
Flowering and fruit-set in cassava under extended red-light photoperiod supplemented with plant-growth regulators and pruning
BMC Plant Biology (2023)
-
Colored Plastic Film Mulching Regulates Light Quality and Sucrose Metabolism in Wine Grape in an Arid Desert Oasis
Journal of Plant Growth Regulation (2023)
-
Bacillus sp. LC390B from the Maize Rhizosphere Improves Plant Biomass, Root Elongation, and Branching and Requires the Phytochromes PHYA and PHYB for Phytostimulation
Journal of Plant Growth Regulation (2023)
-
Spectrum splitting through CuS–ZnO/water hybrid nanofluid for agricultural greenhouse cooling applications: An experimental study
Journal of Thermal Analysis and Calorimetry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.