Abstract
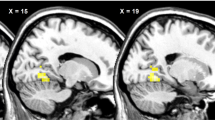
After eating, the human brain senses a biochemical change and then signals satiation, but precisely when this occurs is unknown. Even for well-established physiological systems like glucose–insulin regulation, the timing of interaction between hormonal processes and neural events is inferred mostly from blood sampling1,2,3,4,5,6. Recently, neuroimaging studies have provided in vivo information about the neuroanatomical correlates of the regulation of energy intake7,8,9,10. Temporal orchestration of such systems, however, is crucial to the integration of neuronal and hormonal signals that control eating behaviour11. The challenge of this functional magnetic resonance imaging study is to map not only where but also when the brain will respond after food ingestion. Here we use a temporal clustering analysis technique to demonstrate that eating-related neural activity peaks at two different times with distinct localization. Importantly, the differentiated responses are interacting with an internal signal, the plasma insulin. These results support the concept of temporal parcellation of brain activity12, which reflects the different natures of stimuli and responses. Moreover, this study provides a neuro-imaging basis for detecting dynamic processes without prior knowledge of their timing, such as the acute effects of medication and nutrition in the brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rolls, E. T. & Treves, A. in Neural Network and Brain Function (eds Rolls, E. T. & Treves, A.) 17–22 (Oxford Univ. Press, Oxford/New York, 1998).
Saper, C. B. in The Human Nervous System (ed. Paxinos, G.) 389– 413 (Academic, New York, 1990).
Benzo, C. A. The hypothalamus and blood glucose regulation. Life Sci. 32, 2509–2515 (1983).
Rohner-Jeanrenaud, F., Robbioni, E., Ionescu, E., Sauter, J. F. & Jeanrenaud, B. Central nervous system regulation of insulin secretion. Adv. Metab. Disord. 10, 193–220 (1983).
Oomura, Y. in Handbook of the Hypothalamus (eds Morgane, P. J. & Panksepp, J.) 557–620 (Dekker, New York, 1981).
Grossman, S. P. Role of the hypothalamus in the regulation of food and water intake. Psychol. Rev. 82, 200–224 (1975).
Tataranni, P. A. et al. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl Acad. Sci. USA 96, 4569–4574 (1999).
Gautier, J.-F. et al. Regions of the human brain affected during a liquid-meal taste perception in the fasting state: a positron emission tomography study. Am. J. Clin. Nutr. 70, 806–810 (1999).
Denton, D. et al. Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proc. Natl Acad. Sci. USA 96, 2532–2537 (1999).
Matsuda, M. et al. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 48, 1801– 1806 (1999).
Woods, S. C., Seeley, R. J., Porte, D. J. & Schwartz, M. W. Signals that regulate food intake and energy homeostasis. Science 280, 1378–1383 ( 1998).
Liu, Y., Gao, J.-H., Liotti, M., Pu, Y. & Fox, P. T. Temporal dissociation of parallel processing in the human subcortical outputs. Nature 400, 364–367 (1999).
Friston, K. J., Jezzard, P. & Turner, R. Analysis of functional MRI time-series. Hum. Brain Mapp. 1, 153–171 (1994).
Rosen, B. R., Buckner, R. L. & Dale, A. M. Event-related functional MRI: past, present, and future. Proc. Natl Acad. Sci. USA 95, 773 –780 (1998).
Ngan, S.-C. & Hu, X. Analysis of functional magnetic resonance imaging data using self-organizing mapping with spatial connectivity. Magn. Reson. Med. 41, 939–946 (1999).
Bendettini, P. A., Jesmanowicz, A., Wong, E. C. & Hyde, J. S. Processing strategies for time course data sets in functional MRI of the human brain. Magn. Reson. Med. 30, 161– 173 (1993).
Xiong, J., Gao, J.-H., Lancaster, J. L. & Fox, P. T. Assessment and optimization of functional MRI analyses. Hum. Brain Mapp. 4, 153–167 ( 1996).
Zatorre, R. J., Jones-Gotman, M., Evans, A. C. & Meyer, E. Functional localization and lateralization of human olfactory cortex. Nature 360, 339–340 ( 1992).
Kinomura, S. et al. Functional anatomy of taste perception in the human brain studied with positron emission tomography. Brain Res. 659, 263–266 (1994).
Scott, T. R., Yan, J. & Rolls, E. T. Brain mechanisms of satiety and taste in macaques. Neurobiology 3, 281–292 (1995).
Reiman, E. M. The application of positron emission tomography to the study of normal and pathologic emotions. J. Clin. Psychiatry 58, S4–12 (1997).
Le Doux, J. E. in Handbook of Physiology (eds Plum, F. & Mountcastle, V. B.) 419–459 (Am. Physiol. Soc., Washington, DC, 1987).
Liu, Y. et al. Temporal differentiation of the global effect and regional effect of microwave heating on the monkey cerebral circulation. NeuroImage 9, S120 (1999).
Fox, P. T. et al. Mapping human visual cortex with positron emission tomography. Nature 323, 806–809 (1986).
Fox, P. T., Mintun, M. A., Reiman, E. M. & Raichle, M. E. Enhanced detection of focal brain responses using intersubject averaging and change-distribution analysis of subtracted PET images. J. Cereb. Blood Flow Metab. 8, 642–653 (1988).
Worsley, K. J., Evans, A. C., Marrett, S. & Neelin, P. A three dimensional statistical analysis for CBF activation studies in human brain. J. Cereb. Blood Flow Metab. 12, 900 –918 (1992).
Friston, K. J., Worsley, K. J., Frackowak, R. S. J., Mazziotta, J. C. & Evans, A. C. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping 3, 210–220 (1994).
Worsley, K. J. Local maxima and the expected Euler characteristic of excursion sets of χ2, F and t fields. Adv. Appl. Prob. 26, 13–42 (1994).
Buechel, C., Coull, J. T. & Friston, K. J. The predictive value of changes in effective connectivity for human learning. Science 283, 1538– 1541 (1999).
Liu, H.-L. et al. Comparison of the center of mass and navigator echo corrections of image shift induced by central frequency shift in EPI fMRI. Proc. Int. Soc. Magn. Reson. Med. 7, 271 (1999).
Acknowledgements
We thank M. Matsuda, R. A. DeFronzo, L. Nickerson, S. C. Pridgen and M. Liotti for their help in this study.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, Y., Gao, JH., Liu, HL. et al. The temporal response of the brain after eating revealed by functional MRI. Nature 405, 1058–1062 (2000). https://doi.org/10.1038/35016590
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35016590
This article is cited by
-
Carbohydrate mouth rinse is no more effective than placebo on running endurance of dehydrated and heat acclimated athletes
European Journal of Applied Physiology (2023)
-
Gray matter volume and functional connectivity underlying binge eating in healthy children
Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity (2022)
-
Food intake precipitates seizures in temporal lobe epilepsy
Scientific Reports (2021)
-
Two-Dimensional Temporal Clustering Analysis for Patients with Epilepsy: Detecting Epilepsy-Related Information in EEG-fMRI Concordant, Discordant and Spike-Less Patients
Brain Topography (2018)
-
Effect of mouth rinsing and ingestion of carbohydrate solutions on mood and perceptual responses during exercise
Journal of the International Society of Sports Nutrition (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.