Abstract
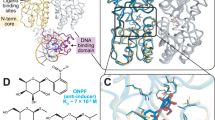
The three-dimensional structure of the 66-amino acid cro repressor protein of bacteriophage λ suggests how it binds to its operator DNA. We propose that a dimer of cro protein is bound to the B-form of DNA with the 2-fold axis of the dimer coincident with the 2-fold axis of DNA. A pair of 2-fold-related α-helices of the represser, lying within successive major grooves of the DNA, seem to be a major determinant in recognition and binding. In addition, the C-terminal residues of the protein, some of which are disordered in the absence of DNA, appear to contribute to the binding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Echols, H. A. Rev. Genet. 6, 157–190 (1972).
Herskowitz, I. A. Rev. Genet. 7, 289–323 (1973).
Ptashne, M. et al. Science 194, 156–161 (1976).
Ptashne, M. et al. Cell 19, 1–11 (1980).
Johnson, A., Meyer, B. J. & Ptashne, M. Proc. natn. Acad. Sci. U.S.A. 75, 1783–1787 (1978).
Takeda, Y. J. molec. Biol. 127, 177–191 (1979).
Folkamanis, A., Takeda, Y., Simuth, J., Gussin, G. & Echols, H. Proc. natn. Acad. Sci. U.S.A. 73, 2249–2253 (1976).
Takeda, Y., Folkmanis, A. & Echols, H. J. biol. Chem. 252, 6177–6183 (1977).
Hsiang, M. W., Cole, R. D., Takeda, Y. & Echols, H. Nature 270, 275–277 (1977).
Roberts, T. M., Shimatake, H., Brady, C. & Rosenburg, M. Nature 270, 274–275 (1977).
Green, D. W., Ingram, V. M. & Perutz, M. F. Proc. R. Soc. A225, 287–307 (1954).
Matthews, B. W. in The Proteins Vol. 3. 3rd edn (eds Neurath, H. & Hill, R. L.) 403–590 (Academic, New York, 1977).
Blundell, T. L. & Johnson, L. N. Protein Crystallography (Academic, London, 1976).
Anderson, W. F., Takeda, Y., Echols, H. & Matthews, B. W. J. molec. Biol. 130, 507–510 (1979).
Anderson, W. F., Takeda, Y., Ohlendorf, D. H. & Matthews, B. W. Proc. 7th Katzir-Katchalsky Conf. on Structural Aspects of Recognition and Assembly in Biological Macromolecules March 1980 (in the press).
Schmid, M. F. et al. Acta crystallogr. (in the press).
Molnar, C. E., Barry, C. D. & Rosenberger, F. U. Tech. Memo. No. 229 (Computer Systems Laboratory, Washington University, St. Louis, 1976).
Blow, D. M. & Crick, F. H. C. Acta crystallogr. 12, 794–802 (1959).
Bricogne, G. Acta crystallogr. A30, 395–405 (1974).
Richards, F. M. J. molec. Biol. 37, 225–230 (1968).
Colman, P. M., Jansonius, J. N. & Matthews, B. W. J. molec. Biol. 70, 701–724 (1972).
Maniatis, T. et al. Cell 5, 109–113 (1975).
Flashman, S. M. Molec. gen. Genet. 166, 61–73 (1978).
Meyer, B. J., Maurer, R. & Ptashne, M. J. molec. Biol. 139, 163–194 (1980).
Watson, J. D. & Crick, F. H. C. Nature 171, 737 (1953).
Arnott, S. & Hukins, D. W. L. Biochem. biophys, Res. Commun. 47, 1504–1509 (1972).
Church, G. M., Sussman, J. L. & Kim, S. -H. Proc. natn. Acad. Sci. U.S.A. 74, 1458–1462 (1977).
Johnson, A., Meyer, B. J. & Ptashne, M. Proc. natn. Acad. Sci. U.S.A. 76, 5061–5065 (1979).
Sung, M. T. & Dixon, G. H. Proc. natn. Acad. Sci. U.S.A. 67, 1616–1623 (1970).
Adler, K. et al. Nature 237, 322–327 (1972).
Warrant, R. W. & Kim, S. H. Nature 271, 130–135 (1978).
McKay, D. & Steitz, T. A. Nature (this issue).
Herskowitz, I. & Hagen, D. A. Rev. Genet. 14, 399–445 (1980).
Echols, H. in The Molecular Genetics of Development (eds Loomis, W. & Leighton, T.) 1–16 (Academic, New York, 1980).
Johnson, A. thesis, Harvard Univ. (1980); and manuscript in preparation.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Anderson, W., Ohlendorf, D., Takeda, Y. et al. Structure of the cro repressor from bacteriophage λ and its interaction with DNA. Nature 290, 754–758 (1981). https://doi.org/10.1038/290754a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/290754a0
This article is cited by
-
DNA–MBF1 study using molecular dynamics simulations
European Biophysics Journal (2021)
-
Computational Simulation of Holin S105 in Membrane Bilayer and Its Dimerization Through a Helix-Turn-Helix Motif
The Journal of Membrane Biology (2021)
-
Structural study to analyze the DNA-binding properties of DsrC protein from the dsr operon of sulfur-oxidizing bacterium Allochromatium vinosum
Journal of Molecular Modeling (2019)
-
Cytochrome c oxidase is activated by the oncoprotein Ras and is required for A549 lung adenocarcinoma growth
Molecular Cancer (2012)
-
Energetics of the protein-DNA-water interaction
BMC Structural Biology (2007)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.