Abstract
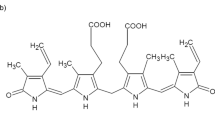
THE biological breakdown of haem proceeds through a series of intermediates of which the orange-yellow bile pigment, bilirubin, the chromophore responsible for coloration in the various forms of jaundice, is a key member. In the adult human, for example, erythrocytes have a lifetime of about 3 months and their destruction produces about 300 mg bilirubin each day1,2. Although there were some early doubts (arising from the possibility of a cyclisation between the CIS vinyl group and the oxygen function at C19)3, the gross chemical structure (Fig. 1) for bilirubin has been beyond reasonable dispute for many years4. There remain, however, considerable structural uncertainties with respect to (1) stereochemistry, the geometry around bridges 5 and 15 being unknown, and represented variously as E or Z in the current literature. (The two configurations, E and Z, have been recognised in a model pyrromethanone system, however, (see ref. 5 and Fig. 2)); (2) tautomerism, although spectroscopic evidence favours the lactam formula for rings A and D (shown in Fig. 1) over the lactim6–9; and (3) conformation, although several speculations, supported by spectroscopic evidence and model making, are available10–17. We report the X-ray analysis of bilirubin—the first such analysis of a naturally occurring linear tetrapyrrole—which shows that bilirubin has the Z configuration at the C4–C5 and C15–C16 bonds, and possesses in the crystal a ridge tile conformation with considerable intramolecular hydrogen bonding.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lathe, G. H., Essays in Biochemistry, 8, 107–148 (CRC, Cleveland, Ohio, 1972).
Schmid, R., and McDonagh, A. F., Ann. N. Y. Acad. Sci., 244, 533–552 (1975).
Fischer, H., and Orth, H., Die Chemie des Pyrrols, 2.1, 626 (Akademische. Verlagsgesellschaft, Leipzig, 1937).
Fischer, H., Plieninger, H., and Weissbarth, O., Hoppe-Seyler's Z. Physiol. Chem., 268, 231–260 (1941).
Falk, H., Grubmayr, K., Herzig, U., and Hofer, O., Tetrahedron Lett., 559–562 (1975).
Gray, C. H., Kulczycka, A., and Nicholson, D. C., J. chem. Soc., 2276–2285 (1961).
Kuenzle, C. C., Biochem. J., 119, 395–409 (1970).
Hutchinson, D. W., Johnson, B., and Knell, A. J., Biochem. J., 123, 483–484 (1971).
Manitto, P., Ricca, G. S., and Monti, D., Gazzetta Chim. Ital., 104, 633–637 (1974).
Nichol, A. W., and Morell, D. B., Biochim. biophys. Acta, 177, 599–609 (1969).
Fog, J., and Jellum, E., Nature, 198, 88–89 (1963).
Fog, J., and Bugge-Asperheim, B., Nature, 203, 756–757 (1964).
Brodersen, R., Flodgaard, H., and Hansen, J. K., Acta scand. Chim., 21, 2284–2285 (1967).
Blauer, G., and King, T. E., J. biol. Chem., 245, 372–381 (1970).
Kuenzle, C. C., Weibel, M. H., Pelloni, R. R., and Hemmerich, P., Biochem. J., 133, 364–368 (1973).
Knell, A. J., Hancock, F., and Hutchinson, D. W., in Metabolism and Chemistry of Bilirubin and Related Tetrapyrrols (edit. by Bakkan, A. F. and Fog, J.), 234–239 (Pediatric Research Institute, Oslo, 1975).
Manitto, P., and Monti, D., J. chem. Soc. Chem. Comm., 112–123 (1976).
Bonnett, R., Hursthouse, M. B., and Neidle, S., J. C. S. Perkin Trans., II, 902–906, 1335–1340 (1972).
Rüdiger, W., Fortschr. Chem. Organ. Natur., 29, 60–139 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
BONNETT, R., DAVIES, J. & HURSTHOUSE, M. Structure of bilirubin. Nature 262, 326–328 (1976). https://doi.org/10.1038/262326a0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/262326a0
This article is cited by
-
Safety assessment and antimalarial property of methanol extract of Fagara zanthoxyloides root-bark on Plasmodium berghei-infected mice
Comparative Clinical Pathology (2021)
-
Bilirubin and its congeners: conformational analysis and chirality from metadynamics and related computational methods
Monatshefte für Chemie - Chemical Monthly (2019)
-
Cycloaddition of [3]dendralene derivatives to dinitrobenzofuroxan and nitrobenzodifuroxan
Chemistry of Heterocyclic Compounds (2015)
-
Bilirubin hydrate
Monatshefte für Chemie - Chemical Monthly (2014)
-
Spectroscopic studies on bilirubin aggregate at liquid/liquid interface
Analytical and Bioanalytical Chemistry (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.