Abstract
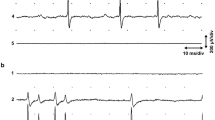
EVIDENCE in favour of the concept of servo control of muscle as expressed by Merton1, depended first on an interpretation of the electromyographic silent period resulting from procedures which unload the spindle2. Direct recording of spindle and fusimotor activity has supported the idea in the case of certain reflex limb movements3 and in the respiratory muscles of anaesthetized animals4,5. We have sought to extend consideration to the jaw muscles of the cat by recording from their afferents during reflex movements. The cell bodies of these first order afferents can be located in the mid-brain nucleus of the fifth cranial nerve (mid-n V) with extra-cellular metal microelectrodes, with a minimum of operative interference6. Receptors from jaw closing muscles greatly outnumber those from the openers7,8 and attention is restricted here to spindles and tendon organs in the former. Three other types of receptor are potentially represented and must be eliminated. First, tooth mechano-receptors are plentiful, particularly related to the canine teeth9. They were distinguished by their sensitivity to direct pressure on a tooth or gum with jaw supported. Cells belonging to such receptors occurred commonly in groups, their frequency range was lower than that of spindles and their extra-cellular action potentials were usually larger than those of any other cells. Second, extra-ocular muscle stretch receptors have been stated to have their cell bodies in the mid-n V10 and, because the bony orbit is deficient in the cat, jaw movements might have excited them. Although units were often found to be affected by passive eye movements, however, they were invariably more sensitive to jaw movement. Third, temporo-mandibular joint receptors might be represented in the mid-n V, but in fact no unit has been encountered which could not be ascribed to one of the other groups. Elimination of the above types leaves spindles and tendon organs in the jaw closing muscles. They can be distinguished as follows. The three muscles act in parallel on the jaw, so that twitch contraction in one of them brings about a pause in a spindle located in any of them, if some jaw movement is permitted. A tendon organ in one muscle will also pause during a twitch in either of the others, but will speed during a twitch of its own muscle. A twitch to each muscle in turn can therefore identify and locate a tendon organ or identify a spindle. The spindle must then be located by probing the muscle surface. Another important test for spindles is their sensitivity to small doses of succinyl choline (SCh) injected intra-arterially11. Each unit recorded was first tested as above (save for the SCh) and then its behaviour studied during active jaw movements. In the cat lightly anaesthetized with sodium pentobarbitone, reflex movements could be produced by putting fluid in the mouth, mechanically stimulating the pharynx and by obstructing the airway. Testing with SCh was delayed till after recording active movements.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Merton, P. A., The Spinal Cord (edit. by G. E. W. Wolstenholme), 247 (Churchill, London, 1953).
Hammond, P. H., Merton, P. A., and Sutton, G. C., Brit. Med. Bull., 12, 214 (1956).
Granit, R., and Kaada, B. R., Acta Physiol. Scand., 27, 130 (1952).
Critchlow, V., and Von Euler, C. J., J. Physiol., 168, 820 (1963).
Sears, T. A., Nature, 197, 1013 (1963).
Davey, M. R., and Taylor, A., J. Physiol., 185, 62P (1966).
Corbin, K. B., and Harrison, F., J. Neurophysiol., 3, 423 (1940).
Jerge, C. R., J. Neurophysiol., 26, 379 (1963).
Corbin, K. B., J. Comp. Neurol., 73, 153 (1940).
Fillenz, M., J. Physiol., 128, 182 (1955).
Granit, R., Skoglund, S., and Thesleff, S., Acta Physiol. Scand., 28, 134 (1953).
Fehr, H-U., J. Physiol., 178, 98 (1965).
Matthews, P. B. C., Physiol. Rev., 44, 219 (1964).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
TAYLOR, A., DAVEY, M. Behaviour of Jaw Muscle Stretch Receptors during Active and Passive Movements in the Cat. Nature 220, 301–302 (1968). https://doi.org/10.1038/220301a0
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1038/220301a0
This article is cited by
-
A novel myosin present in cat jaw-closing muscles
Journal of Muscle Research and Cell Motility (1981)
-
Role of different types of fibers of the infraorbital nerve in synaptic activation of masseter and digastric motoneurons
Neurophysiology (1981)
-
Histochemical profiles of rat soleus intrafusal fibres after chronic exercise
The Histochemical Journal (1975)
-
Muscle receptors in the control of voluntary movement
Spinal Cord (1972)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.