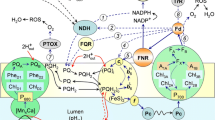
Abstract
Regulatory reactions providing the photosynthetic apparatus with the ability to respond to variations of irradiance by changes in activities of the light and the dark stages of photosynthesis within a time range of seconds and minutes are considered in the review. At the light stage, such reactions are related to the changes in both distribution of light energy between two photosystems and probability of nonphotochemical dissipation of absorbed quanta in PSI and PSII. These regulatory reactions operate in a negative feedback mode, thus avoiding overreduction of electron transport chain and minimizing the probability of generation of reactive oxygen species. The crucial role in preventing the generation of reactive oxygen species belongs to dynamic regulation of electron transport activity despite the presence of complex system of their detoxification in chloroplasts. In dark reactions of Calvin cycle, the regulatory responses involve a positive feedback principle being related to redox regulation of activities of several enzymes, which is sensitive to the reduction status of PSI acceptor side. The complex of regulatory reactions based on negative and positive feedback principles provides prolonged functioning of a chloroplast and high stability of photosynthetic activity under various light conditions.
Similar content being viewed by others
REFERENCES
Weston, E., Thorogood, K., Vinti, G., and López-Jues, E., Light Quantity Controls Leaf-Cell and Chloroplast Development in Arabidopsis thaliana Wild Type and Blue-Light-Perception Mutants, Planta, 2000, vol. 211, pp. 807–815.
Anderson, J M., Photoregulation of the Composition, Function, and Structure of Thylakoid Membranes, Annu. Rev. Plant Physiol., 1986, vol. 37, pp. 93–136.
Critchley, C., Molecular Adaptation to Irradiance: The Dual Functionality of Photosystem II, Concepts in Photobiology: Photosynthesis and Photomorphogenesis, Singhal, G.S. et al., <nt>Eds.</nt>, New Delhi: Narosa Publ., 1999, pp. 572–587.
Escoubas, J.M., Lomas, M., LaRoche, J., and Falkowski, P.G., Light Intensity Regulation of cab Gene Transcription Is Signaled by the Redox State of the Plastoquinone Pool, Proc. Natl. Acad. Sci. USA, 1995, vol. 92, pp. 10237–10241.
Chazdon, R.L. and Pearcy, R.W., Photosynthetic Responses to Light Variation in Rain Forest Species: 1. Induction under Constant and Fluctuating Light Conditions, Oecologia, 1986, vol. 69, pp. 517–523.
Pearcy, R.W., Roden, J.S., and Gamon, J.A., Sunfleck Dynamics in Relation to Canopy Structure in a Soybean (Glycine max (L.) Merr.) Canopy, Agric. Forest Meteorol., 1990, vol. 52, pp. 359–372.
Doring, G., Renger, G., Vater, J., and Witt, H.T., Properties of Photoactive Chlorophyll a II in Photosynthesis, Z. Naturforsch., 1969, vol. 24b, pp. 1139–1143.
Krau, N., Ninrich, W., Witt, I., Fromme, P., Pritzkow, W., Dauterb, Z., Betzel, C., Wilson, K.S., Witt, H.T., and Saenger, W., Three-Dimensional Structure of System I of Photosynthesis at 6A Resolution, Nature, 1996, vol. 361, pp. 326–331.
Klimov, V.V., Dolan, E., and Ke, B., EPR Properties of an Intermediary Electron Acceptor (Pheophytin) in Photosystem II Reaction Centers at Cryogenic Temperatures, FEBS Lett., 1980, vol. 112, pp. 97–100.
Hastings, G., Hoshina, S., Webber, A.N., and Blankenship, R.E., Universality of Energy and Electron Transfer Processes in Photosystem I, Biochemistry, 1995, vol. 34, pp. 15 512–15 522.
Van Gorkom, H.J., Identification of the Reduced Primary Electron Acceptor of Photosystem II as a Bound Semiquinone Anion, Biochim. Biophys. Acta, 1974, vol. 347, pp. 439–442.
Bendall, D.S., Photosynthetic Cytochromes of Oxygenic Organisms, Biochim. Biophys. Acta, 1982, vol. 683, pp. 119–151.
Hauska, G., Hart, E., Gabellini, N., and Lockau, W., Comparative Aspects of Quinol–Cytochrome c/Plastocyanin Oxidoreductases, Biochim. Biophys. Acta, 1983, vol. 726, pp. 97–133.
Heathcote, P., Moenne-Loccoz, P., Rigby, S.E.J., and Evans, M.C.W., Photoaccumulation in Photosystem I Does Produce a Phylloquinone (A1 - ) Radical, Biochemistry, 1996, vol. 35, pp. 6644–6650.
Golbeck, J.H., Structure and Function of Photosystem I, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1992, vol. 43, pp. 293–324.
Mitchell, P., Chemiosmotic Coupling in Oxidative and Photosynthetic Phosphorylation, Biol. Rev., 1966, vol. 41, pp. 445–502.
Seibert, M., Biochemical, Biophysical, and Structural Characterization of the Isolated Photosystem II Reaction Center Complex, The Photosynthetic Reaction Center, vol. 1, Deisenhofer, J. and Norris, J.P., <nt>Eds.</nt>, San Diego: Academic, 1993, pp. 319–356.
Peter, G.F. and Thornber, J.P., Biochemical Composition and Organization of Higher Plant Photosystem II Light-Harvesting Pigment Proteins, J. Biol. Chem., 1991, vol. 266, pp. 16 745–16.
Farquhar, G.D., von Cammerer, S., and Berry, J.A., A Bio-chemical Model of Photosynthetic CO2 Assimilation in Leaves of C3 Species, Planta, 1980, vol. 149, pp. 78–90.
Backhausen, J.E., Kitzmann, C., and Scheibe, R., Competition between Electron Acceptors in Photosynthesis: Regulation of the Malate Valve during CO2 Fixation and Nitrite Reduction, Photosynth. Res., 1994, vol. 42, pp. 75–86.
Genty, B., Briantais, J.-M., and Baker, N.R., The Relationship between the Quantum Yield of Photosynthetic Electron Transport and Quenching of Chlorophyll Fluorescence, Biochim. Biophys. Acta, 1989, vol. 990, pp. 87–92.
Genty, B., Harbinson, J., and Baker, N.R., Relative Quantum Efficiencies of the Two Photosystems of Leaves in Photorespiratory and Non-Photorespiratory Conditions, Plant Physiol. Biochem., 1990, vol. 28, pp. 1–10.
Genty, B., Harbinson, J., Briantais, J.-M., and Baker, N.R., The Relationship between Non-Photochemical Quenching of Chlorophyll Fluorescence and the Rate of Photosystem 2 Photochemistry in Leaves, Photosynth. Res., 1990, vol. 25, pp. 249–257.
Harbinson, J., The Responses of Thylakoid Electron and Light Utilization Efficiency to Sink Limitation of Photosynthesis, Photoinhibition of Photosynthesis: From Molecular Mechanims to the Field, Baker, N.R. and Bowyer, J.R., <nt>Eds.</nt>, Oxford: Bios Sci., 1994, pp. 273–295.
Bukhov, N.G., Makarova, V.V., and Krendeleva, T.E., Coordinated Changes in the Redox State of Photosys-tems I and II in Sunflower Leaves at Different Irradiances, Fiziol. Rast., 1998, vol. 45, pp. 551–557 (Russ. J. Plant Physiol., Engl. Transl.).
Macpherson, A.N., Telfer, A., Barber, J., and Truscott, T.G., Direct Detection of Singlet Oxygen from Isolated Photosystem II Reaction Centers, Biochim. Biophys. Acta, 1993, vol. 1143, pp. 301–309.
Takahashi, M. and Asada, K., Superoxide Production in Aprotic Interior of Chloroplast Thylakoids, Arch. Biochem. Biophys., 1988, vol. 267, pp. 714–722.
McCord, J.M. and Fridovich, I., Superoxide Dismutase: An Enzymic Function for Erthyrocuprein (Hemocuprein), J. Biol. Chem., 1969, vol. 244, pp. 6049–6055.
Asada, K., Mechanisms for Scavenging Reactive Mole-cules Generated in Chloroplasts under Light Stress, Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field, Baker, N.R. and Bowyer, J.R., Oxford: Bios Sci., 1994, pp. 129–142.
Ohad, I., Koike, H., Shochat, S., and Inoue, Y., Changes in the Properties of Reaction Center II during the Initial Stages of Photoinhibition as Revealed by Thermolumi-nescence Measurements, Biochim. Biophys. Acta, 1988, vol. 933, pp. 288–298.
Nedbal, L., Masojidek, J., Komenada, J., Prasil, O., and Šetlik, I., Three Types of PSII Photoinhibition: 2. Slow Processes, Photosynth. Res., 1990, vol. 24, pp. 89–97.
Richter, M., Rhüle, W., and Wild, A., Studies on the Mechanism of Photosystem II Photoinhibition: 2. The Involvement of Toxic Oxygen Species, Photosynth. Res., 1990, vol. 24, pp. 237–243.
Šetlik, I., Allakhverdiev, S.I., Nedbal, L., Setlikova, E., and Klimov, V.V., Three Types of Photosystem II Photoinactivation, Photosynth. Res., 1990, vol. 23, pp. 39–48.
Krieger, A. and Weis, E., Energy-Dependent Quenching of Chlorophyll-a-Fluorescence, the Involvement of Proton– Calcium Exchange at Photosystem II, Photosynthetica, 1992, vol. 27, pp. 89–98.
Ono, T. and Inoue, Y., Removal of Ca2+ by pH 3.0 Treatment Inhibits S2 to S3 Transition in Photosynthetic Oxygen Evolution System, Biochim. Biophys. Acta, 1989, vol. 973, pp. 443–449.
Neubauer, C. and Yamamoto, H.Y., Mehler-Peroxidase Reaction Mediates Zeaxanthin Formation and Zeaxanthin-Related Fluorescence Quenching in Intact Chloroplasts, Plant Physiol., 1992, vol. 99, pp. 1354–1361.
Jablonski, P.P. and Anderson, J.W., Light-Dependent Reduction of Hydrogen Peroxide by Ruptured Pea Chloroplasts, Plant Physiol., 1982, vol. 69, pp. 1407–1413.
Miyake, C. and Asada, K., Thylakoid-Bound Ascorbate Peroxidase in Spinach Chloroplasts and Photoreduction of Its Primary Oxidation Product Monodehydroascorbate Radicals in Thylakoids, Plant Cell Physiol., 1992, vol. 33, pp. 541–553.
Hossain, H.A., Nakano, Y., and Asada, K., Monodehydroascorbate Reductase in Spinach Chloroplasts and Its Participation in Regeneration of Ascorbate for Scavenging Hydrogen Peroxide, Plant Cell Physiol., 1984, vol. 25, pp. 385–395.
Hossain, H.A. and Asada, K., Purification of Dehydroascorbate Reductase from Spinach and Its Characterization as a Thiol Enzyme, Plant Cell Physiol., 1984, vol. 25, pp. 85–95.
Smith, I.K., Vierheller, T.L., and Thorne, C.A., Properties and Function of Glutathione Reductase in Plants, Physiol. Plant., 1989, vol. 77, pp. 449–456.
Fryer, M.J., The Antioxidant Effects of Thylakoid Vitamin E α-Tocopherol), Plant Cell Environ., 1992, vol. 15, pp. 381–392.
Van Hasselt, P.R., de Kok, C.J., and Kuiper, P.J., Effect of α-Tocopherol, β-Carotene, Monogalactosyldiglyceride and Phosphatidylcholine on Light-Induced Degradation of Chlorophyll in Acetone, Physiol. Plant., 1979, vol. 45, pp. 475–479.
Bonaventura, C. and Myers, J., Fluorescence and Oxygen Evolution from Chlorella pyrenoidosa, Biochim. Biophys. Acta, 1969, vol. 189, pp. 366–383.
Bennett, J., Protein Phosphorylation in Green Plant Chloroplast, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1991, vol. 42, pp. 281–311.
Allen, J.F., Protein Phosphorylation in Regulation of Photosynthesis, Biochim. Biophys. Acta, 1992, vol. 1098, pp. 275–335.
Forsberg, J. and Allen, J.F., Protein Tyrosine Phosphorylation in the Transition to Light State 2 of Chloroplast Thylakoids, Photosynth. Res., 2001, vol. 68, pp. 71–79.
Zito, F., Finazzi, G., Delosme, R., Nitschke, W., Picot, D., and Wollman, F.A., The Q0 Site of Cytochrome b 6 f Complex Controls the Activation of the LHCII Kinase, EMBO J., 1999, vol. 18, pp. 2961–2969.
Vener, A.V., van Kan, P.J., Rich, P.R., Ohad, I., and Andersson, B., Plastoquinol at the Quinol Oxidation Site of Reduced Cytochrome bf Mediates Signal Transduction between Light and Protein Phosphorylation: Thylakoid Protein Kinase Deactivated by a Single Turnover Flash, Proc. Natl. Acad. Sci. USA, 1997, vol. 94, pp. 1585–1590.
Chow, W.S., Telfer, A., Chapman, D.J., and Barber, J., State 1–State 2 Transition in Leaves and Its Association with ATP-Induced Chlorophyll Fluorescence Quenching, Biochim. Biophys. Acta, 1981, vol. 638, pp. 60–68.
Horton, P. and Black, M.T., Light-Dependent Quenching of Chlorophyll Fluorescence in Pea Chloroplasts Induced by Adenosine-5'-Triphosphate, Biochim. Biophys. Acta, 1981, vol. 635, pp. 53–62.
Bukhov, N.G., Wiese, C., Neimanis, S., and Heber, U., Control of Photosystem II in Spinach Leaves by Continuous Light and by Light Given in the Dark, Photosynth. Res., 1996, vol. 50, pp. 181–191.
Satoh, K., Fluorescence Induction and Activity of Ferredoxin-NADP + Reductase in Bryopsis Chloroplasts, Biochim. Biophys. Acta, 1981, vol. 638, pp. 327–331.
Horton, P. and Hague, A., Studies on the Induction of Chlorophyll Fluorescence in Isolated Barley Protoplasts: 4. Resolution of Non-Photochemical Quenching, Biochim. Biophys. Acta, 1988, vol. 932, pp. 107–115.
Quick, W.P. and Stitt, M., An Examination of Factors Contributing to Non-Photochemical Quenching of Chlorophyll Fluorescence in Barley Leaves, Biochim. Biophys. Acta, 1989, vol. 977, pp. 287–296.
Delosme, R., Beal, D., and Joliot, P., Photoacoustic Detection of Flash-Induced Charge Separation in Photosynthetic Systems: Spectral Dependence of the Quantum Yield, Biochim. Biophys. Acta, 1994, vol. 1185, pp. 56–64.
Delosme, R., Olive, J., and Wollman, F.A., Changes in Light Energy Distribution upon State Transition: An In Vivo Photoacoustic Study of the Wild Type and Photosynthesis Mutants from Chlamydomonas reinhardtii, Biochim. Biophys. Acta, 1996, vol. 1273, pp. 150–158.
Sapozhnikov, D.I., Chemical Structure of Carotenoids and Their Transformation in Plant Cells, Usp. Sovrem. Biol., 1967, vol. 64, pp. 248–258.
Yamamoto, H.Y., Biochemistry of the Xanthophyll Cycle in Higher Plants, Pure Appl. Chem., 1979, vol. 51, pp. 639–648.
Demmig-Adams, B., Gilmore, A., and Adams, W.W., In Vivo Functions of Carotenoids in Plants, FASEB J., 1996, vol. 10, pp. 404–412.
Gilmore, A., Mohanty, N., and Yamamoto, H.Y., Epoxidation of Zeaxanthin and Antheraxanthin Reverses Non-Photochemical Quenching of Photosystem II Chlorophyll a Fluorescence in the Presence of a Transthylakoid pH, FEBS Lett., 1994, vol. 350, pp. 271–274.
Crofts, A. and Yerkes, C.T., A Molecular Mechanism for qE-Quenching, FEBS Lett., 1994, vol. 352, pp. 265–270.
Walters, R.G., Ruban, A.V., and Horton, P., Higher Plant Light-Harvesting Complexes LHCIIa and LHCIIc Are Bound by Dicyclohexylcarbodiimide during Inhibition of Energy Dissipation, Eur. J. Biochem., 1994, vol. 226, pp. 1063–1069.
Gilmore, A. and Yamamoto, H.Y., Linear Model Relating Xanthophylls and Lumen Acidity to Non-Photo-chemical Fluorescence Quenching: Evidence that Antheraxanthin Explains Zeaxanthin-Independent Quenching, Photosynth. Res., 1993, vol. 35, pp. 67–78.
Bukhov, N.G., Kopecky, J., Pfundel, E.E., Klughammer, C., and Heber, U., A Few Molecules of Zeaxanthin per Reaction Centre of Photosystem II Permit Effective Thermal Dissipation of Light Energy in Photosystems II of a Poikilohydric Moss, Planta, 2001, vol. 212, pp. 739–748.
Demmig-Adams, B., Carotenoids and Photoprotection in Plants: A Role for the Xanthophyll Zeaxanthin, Biochim. Biophys. Act a, 1990, vol. 1020, pp. 1–24.
Gilmore, A., Mechanistic Aspects of Xanthophyll-Cycle-Dependent Photoprotection in Higher Plant Chloroplasts and Leaves, Physiol. Plant., 1997, vol. 99, pp. 197–209.
Kohler, B.E., Spangler, C., and Westerfield, C., The 21 Ag State in the Linear Polyene 2,4,6,8,0,12,14,16-Octadeca-octaene, J. Chem. Phys., 1988, vol. 89, pp. 5422–5428.
Owens, T.G., Alberte, R.S., and Gallagher, J.C., Photosynthetic Light-Harvesting Function of Violaxanthin in Nannochlopsis spp. (Eustigmatophyseae), J. Phycol., 1987, vol. 23, pp. 79–85.
DeCoster, B., Christensen, R.I., Gebhard, R., Lugtenburg, J., Farhoosh, R., and Frank, H.A., Low Lying Electronic States of Carotenoids, Biochim. Biophys. Acta, 1992, vol. 1102, pp. 107–119.
Owens, T., Shreve, A.P., and Albrecht, A.C., Dynamics and Mechanism of Singlet Energy Transfer between Carotenoids and Chlorophylls: Light Harvesting and Non-Photochemical Quenching, Research in Photosynthesis, Murata, N., <nt>Ed.</nt>, Dordrecht: Kluwer, 1993, vol. 1, pp. 179–186.
Frank, H.A., Cua, A., Chynwat, V., Young, A., Gosztola, D., and Wasielewski, M.R., Photophysics of the Carotenoids Associated with the Xanthophyll Cycle in Photosynthesis, Photosynth. Res., 1994, vol. 41, pp. 389–395.
Bruce, D., Samson, G., and Carpenter, C., The Origins of Non-Photochemical Quenching of Chlorophyll Fluorescence in Photosynthesis: Direct Quenching by P680 + in Photosystem II Enriched Membranes at Low pH, Biochemistry, 1997, vol. 36, pp. 749–755.
Conjeaud, H., Mathis, P., and Paillotin, G., Primary and Secondary Electron Donors in Photosystem II of Chloroplasts: Rates of Electron Transfer and Location in the Membrane, Biochim. Biophys. Acta, 1979, vol. 546, pp. 280–291.
Weis, E. and Berry, J.A., Quantum Efficiency of Photosystem II in Relation to Energy-Dependent Quenching of Chlorophyll Fluorescence, Biochim. Biophys. Acta, 1987, vol. 894, pp. 198–208.
Krieger, A., Rutherford, A.W., and Jegerschold, C., Thermoluminescence Measurements on Chloride-Depleted and Calcium-Depleted Photosystem II, Biochim. Biophys. Acta, 1998, vol. 1364, pp. 46–54.
Bukhov, N.G., Heber, U., Wiese, C., and Shuvalov, V.A., Energy Dissipation in Photosynthesis: Does the Quenching of Chlorophyll Fluorescence Originates from Antenna Complexes of Photosystem II or from the Reaction Center?, Planta, 2001, vol. 212, pp. 749–758.
Vernotte, C., Etienne, A.L., and Briantais, J.-M., Quenching of the System II Chlorophyll Fluorescence by the Plastoquinone Pool, Biochim. Biophys. Acta, 1979, vol. 545, pp. 519–527.
Bukhov, N.G., Sridharan, G., Egorova, E.A., and Carpentier, R., Interaction of Exogenous Quinones with Membranes of Higher Plant Chloroplasts: Modulation of Quinone Capacities as Photochemical and Non-Photo-chemical Quenchers of Energy in Photosystem II during Light–Dark Transitions, Biochim. Biophys. Acta, 2003, vol. 1604, pp. 115–123.
Rajagopal, S., Egorova, E.A., Bukhov, N.G., and Carpentier, R., Quenching of Excited States of Chlorophyll Molecules in Submembrane Fractions of Photosystem I by Exogenous Quinones, Biochim. Biophys. Acta, 2003, vol. 1606, pp. 147–152.
Aro, E.-M., Virgin, I., and Anderson, B., Photoinhibition of Photosystem II: Inactivation, Protein Damage, and Turnover, Plant Physiol., 1993, vol. 103, pp. 835–843.
Rajagopal, S., Bukhov, N.G., and Carpentier, R., Photo-inhibitory Light-Induced Changes in the Composition of Chlorophyll–Protein Complexes and Photochemical Activity in Photosystem-I Submembrane Fractions, Photochem. Photobiol., 2003, vol. 77, pp. 284–291.
Sonoike, K., Terashima, I., Iwaki, M., and Itoh, S., Destruction of Photosystem I Iron-Sulfur Centers in Leaves of Cucumis sativus L. by Weak Illumination at Chilling Temperatures, FEBS Lett., 1995, vol. 362, pp. 235–238.
Bukhov, N.G., Rajagopal, S., and Carpentier, R., Characterization of P700 as a Photochemical Quencher in Isolated Photosystem I Particles Using Simultaneous Measurements of Absorbance Changes at 830 nm and Photoacoustic Signal, Photosynth. Res., 2002, vol. 74, pp. 295–302.
Bukhov, N.G. and Carpentier, R., Measurements of Photochemical Quenching of Absorbed Quanta in Photosystem I of Intact Leaves Using Simultaneous Measurements of Absorbance Changes at 830 nm and Thermal Dissipation, Planta, 2003, vol. 216, pp. 630–638.
Rajagopal, S., Bukhov, N.G., Tajmir-Riah, A.-T., and Carpentier, R., Control of Energy Dissipation and Photochemical Activity in Photosystem I by NADP-Dependent Reversible Conformational Changes, Biochemistry, 2003, vol. 42, pp. 11 839–11 845.
Buchanan, B.B., Role of Light in the Regulation of Chloroplast Enzymes, Annu. Rev. Plant Physiol., 1980, vol. 31, pp. 341–374.
Buchanan, B.B., Regulation of CO2 Assimilation in Oxygen Photosynthesis: The Ferredoxin/Thioredoxin System, Arch. Biochem. Biophys., 1991, vol. 288, pp. 1–9.
Buchanan, B.B., Carbon Dioxide Assimilation in Oxygenic and Anoxygenic Photosynthesis, Photosynth. Res., 1992, vol. 33, pp. 147–162.
Foyer, C., Furbank, R., Harbinson, J., and Horton, P., Mechanisms Contributing to Photosynthetic Control of Electron Transport by Carbon Assimilation in Leaves, Photosynth. Res., 1990, vol. 25, pp. 83–100.
Schurmann, P., Stritt-Etter, A.-L., and Junsheng, L., Reduction of Ferredoxin:Thioredoxin Reductase by Artificial Electron Donors, Photosynth. Res., 1995, vol. 46, pp. 309–312.
Salamon, Z., Tollin, G., Hirasawa, M., Knaff, D.B., and Schurmann, P., The Oxidation–Reduction Properties of Spinach Thioredoxins f and m and of Ferredoxin:Thioredoxin Reductase, Biochim. Biophys. Acta, 1995, vol. 1230, pp. 114–118.
Knaff, D.R. and Hirasawa, M., Ferredoxin-Dependent Chloroplast Enzymes, Biochim. Biophys. Acta, 1991, vol. 1056, pp. 93–125.
De Pascalis, A.R., Schurmann, P., and Bosshard, H.R., Comparison of the Binding Sites of Plant Ferredoxin for Two Ferredoxin-Dependent Enzymes, FEBS Lett., 1994, vol. 337, pp. 217–220.
Scheibe, R., Redox-Modulation of Chloroplast Enzymes, Plant Physiol., 1991, vol. 96, pp. 1–3.
Dietz, K.J. and Heber, U., Light and CO2 Limitation of Photosynthesis and States of the Reactions Regenerating Ribulose 1,5-Bisphosphate or Reducing Phosphoglycerate, Biochim. Biophys. Acta, 1986, vol. 848, pp. 392–401.
Heber, U., Neimanis, S., Dietz, K.J., and Viil, J., Assimilation Power as a Driving Force in Photosynthesis, Biochim. Biophys. Acta, 1986, vol. 852, pp. 144–155.
Harbinson, J. and Hedley, C.L., The Kinetics of P700 + Reduction: A Novel In Situ Probe of Thylakoid Functioning, Plant Cell Environ., 1989, vol. 12, pp. 357–369.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bukhov, N.G. Dynamic Light Regulation of Photosynthesis (A Review). Russian Journal of Plant Physiology 51, 742–753 (2004). https://doi.org/10.1023/B:RUPP.0000047822.66925.bf
Issue Date:
DOI: https://doi.org/10.1023/B:RUPP.0000047822.66925.bf