Abstract
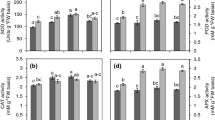
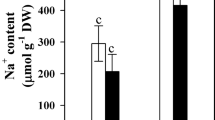
Calcium may be involved in plant tolerance to water deficit by regulating antioxidant metabolism or/and water relations. This study was designed to examine whether external Ca2+ would stimulate drought tolerance in cultured liquorice cells. Water stress induced by 15% PEG significantly reduced fresh weight and relative water content in liquorice cells, but external Ca2+ markedly increased them after stress for 7 days. The activities of catalase (CAT), superoxide dismutase (SOD) declined and activity of peroxidase (POD) slowly increased during water stress imposition. External calcium significantly enhanced SOD and CAT activities, but the effect on POD activity was weak. The effect of external Ca2+ on water deficit tolerance in liquorice cells was not due to the osmotic adjustment in culture medium. Under nonstress conditions, external calcium slightly increased the activities of SOD, CAT, and POD. Ca2+ signal in liquorice cells may be different under stress and nonstress conditions. Under water stress, Ca2+ signal involves in reactive oxygen species transduction pathway and affects the processes participating in regulation of antioxidative enzymes; under nonstress conditions, Ca2+ signal coming from external calcium might not participate in ROS signal transduction pathway resulting in antioxidative defense response in liquorice cells. Less malondialdehyde was accumulated after water stress for 7 days in Ca2+-treated cells than in untreated cells. It was proposed that external calcium could reduce the damage of water deficit and stimulate tolerance to it in liquorice cells by mitigating oxidative stress.
Similar content being viewed by others
REFERENCES
Cooke, A., Cookson, A., and Ealnshaw, M.J., The Mechanism of Action of Calcium in the Inhibition of High Temperature-Induced Leakage of Betacyanin from Beet Root Discs,New Phytol., 1986, vol. 102, pp. 491–497.
Arora, R. and Palta, J. P., In vivoPerturbation of Membrane-Associated Calcium by Freeze-Thaw Stress in Onion Bulb Cells, Plant Physiol., 1988, vol. 87, pp. 622–628.
Bowler, C. and Fluhr, B., The Role of Calcium and Activated Oxygens as Signals for Controlling Cross-Tolerance, Trends Plant Sci., 2000, vol. 5, pp. 241–243.
Cramer, G.R., Lauchli, A., and Polito, V.S., Displacement of Ca2+ by Na+ from the Plasmalemma of Root Cells: A Primary Response to Salt Stress? Plant Physiol., 1985, vol. 79, pp. 207–211.
Dhindsa, R.S. and Matowe, W., Drought Tolerance in Two Mosses Correlated with Enzymatic Defence against Lipid Peroxidation, J. Exp. Bot., 1981, vol. 32, pp. 79–91.
Trippi, V.S., Gidrol, X., and Pradet, A., Effects of Oxidative Stress Caused by Oxygen and Hydrogen Peroxide on Energy Metabolism and Senescence in Oat Leaves, Plant Cell Physiol., 1989, vol. 30, pp. 210–217.
Elstner, E.F., Oxygen Activation and Oxygen Toxicity, Annu. Rev. Plant Physiol., 1982, vol. 33, pp. 73–96.
Smirnoff, N., Antioxidant Systems and Plant Response to the Environment, Environment and Plant Metabolism: Flexibility and Acclimation, Smirnoff, N., Ed., Oxford: Bios Sci., 1995, pp. 217–243.
Asada, K. and Takahashi, M., Production and Scavenging of Reactive Oxygen in Photosynthesis, Photoinhibition, Kyle, D.J., Osmond, C.B., and Arntzen, C.J., Eds., Amsterdam: Elsevier, 1987, pp. 227–287.
Schaedle, M. and Bassham, J., Chloroplast Glutathione Reductase, Plant Physiol., 1977, vol. 59, pp. 1011–1012.
Wang, G.X., Yang, C.D., and Liang, H.G., Changes in SOD Activity and MDA Content during the Development of Broadbean Leaves,Acta Phytophysiol. Sin., 1989, vol. 15, pp. 13–17.
Li, M. and Wang, G.X., Effect of Drought Stress on Activities of Cell Defense Enzymes and Lipid Peroxidation in Glycyrrhiza uralensisSeedlings, Acta Ecol. Sin., 2002, vol. 22, pp. 503–507.
Gao, X. Y., Yang, G.P., Xu, Z.Q., and Xu, F.C., Effect of Calcium on Antioxidant Enzymes of Lipid Peroxidation of Soybean Leaves under Water Stress, J. South China Agric. Univ., 1999, vol. 2, pp. 58–62.
Shu, M.Y. and Fan, M.Q., Effect of Osmotic Stress and Calcium on Membrane-Lipid Peroxidation and the Activity of Defense Enzymes in Fir Seedling, Forest Res., 2000, vol. 4, pp. 391–396.
Qiou, M.X., Liou, J.Q., Shi, Q.H., and Yu, Y.J., Liquorice Community, Vegetation in Middle Part Desert Region of China,Qiou, M.X., Ed., Lanzhou: Gansu Cultur., 2000, pp. 52–53.
Wang, G.X., Zhang, J., Liao, J.X., and Wang, J.L., Hydropassive Evidence and Effective Factors in Stomatal Oscillations of Glycyrrhiza inflataunder Desert Conditions, Plant Sci., 2001, vol. 160, pp. 1007–1013.
Zhang, J., Yao, J., Ding, L., Guo, S.J., and Yang, Y.L., Study Advances on the Utilization of Glycyrrhiza, Grassland and Turf, 2000, vol. 89, pp. 12–17.
Handa, A.K., Bressan, R.A., Handa, S., and Hasegawa, P.M., Characteristics of Cultured Tomato Cells after Prolonged Exposure to Medium Containing Polyethylene Glycol, Plant Physiol., 1982, vol. 69, pp. 514–521.
Chance, B. and Maehly, A.C., Assay of Catalases and Peroxidases, Methods Enzymol., 1955, vol. 2, pp. 764–775.
Handa, A.K., Bressan, R.A., Handa, S., and Hasegawa, P.M., Clonal Variation for Tolerance to Polyethylene Glycol-Induced Water Stress in Cultured Tomato Cells, Plant Physiol., 1983, vol. 72, pp. 645–653.
Giannopolitis, C. N. and Ries, S.K., Superoxide Dismutases: 1. Occurrence in Higher Plants, Plant Physiol., 1977, vol. 59, pp. 309–314.
Zhang, J., Li, J., Cui, S., and Wei, J., Response of Cell Protective Enzymes in Corn Leaf to Water Stress at Seedling Stage, Acta Agric. Boreali-Sin. (Suppl.), 1990, vol. 5, pp. 19–23.
Blum, A., Osmotic Adjustment and Growth of Barley Genotypes under Drought Stress, Crop Sci., 1989, vol. 29, pp. 230–233.
Blum, A. and Sullivan, C.Y., The Comparative Drought Resistance of Landraces of Sorghum and Millet from Dry and Humid Regions, Ann. Bot., 1986, vol. 57, pp. 838–846.
Jiang, Y. and Bingru, H., Effects of Calcium on Antioxidant Activities and Water Relations Associated with Heat Tolerance in Two Cool-Season Grasses, J. Exp. Bot., 2001, vol. 52, pp. 341–349.
Bush, D.S., Calcium Regulation in Plant Cells and Its Role in Signaling, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1995, vol. 46, pp. 95–122.
Hepler, P.K. and Wayne, R.O., Calcium and Plant Development, Annu. Rev. Plant Physiol., 1985, vol. 36, pp. 397-341.
Shinozaki, K. and Yamaguchi-Shinozaki, K., Gene Expression and Signal Transduction in Water-Stress Response, Plant Physiol., 1997, vol. 115, pp. 327–334.
John, G.S., Oxygen Stress and Superoxide Dismutases, Plant Physiol.,1993, vol. 101, pp. 7–12.
Dwivedi, S., Kar, M., and Mishra, D., Biochemical Changes in Excised Leaves of Oryza sativaSubjected to Water Stress, Physiol. Plant., 1979, vol. 45, pp. 35–40.
Mukherjee, S.P. and Choudhwri, M.A., Implication of Hydrogen Peroxide-Ascorbate System on Membrane Permeability of Water Stressed VignaSeedlings, New Phytol., 1985, vol. 99, pp. 355–360.
Quartacci, M.F. and Navari-Izzo, F., Water Stress and Free Radical Mediated Changes in Sunflower Seedlings, J. Plant Physiol., 1992, vol. 139, pp. 621–626.
Badiani, M., de Biasi, M.G., Colognola, M., and Artemi, F., Catalase, Peroxidase and Superoxide Dismutase Activities in Seedlings Submitted to Increasing Water Deficit, Agrochimia, 1990, vol. 34, pp. 90–102.
Siegel, B.Z., Plant Peroxidases-an Organismic Perspective, Plant Growth Regul., 1993, vol. 12, pp. 303–312.
Smith, M.A.L., Spomer, A.L., and Skiles, E.S., Cell Osmolarity Adjustment in Lycopersiconin Response to Stress Pretreatments, J. Plant Nutr., 1989, vol. 12, pp. 233–244.
Ludlow, M.M., Santamaria, J.M., and Fukai, S., Contribution of Osmotic Adjustment to Grain Yields in Sorghum bicolor(L.) Moench under Water-Limited Conditions: 2. Water Stress after Anthesis, Aust. J. Agric. Res., 1990, vol. 41, pp. 67–78.
Dolmetsch, R.E., Lewis, R.S., Goodnow, C.C., and Healy, J.I., Differential Activation of Transcription Factors Induced by Ca2+ Response Amplitude and Duration, Nature, 1997, vol. 386, pp. 855–858.
Knight, H., Brandt, S., and Knight, M.R., A History of Stress Alters Drought Calcium Signaling Pathways in Arabidopsis, Plant J., 1998, vol.16, pp. 681–687.
Knight, H., Trewavas, A. J., and Knight, M.R., Calcium Signaling in Arabidopsis thalianaResponding to Drought and Salinity, Plant J., 1997, vol. 12, pp. 1067–1078.
Gong, M., Chen, S.N., Song, Y.Q., and Li, Z.G., Effects of Calcium and Calmodulin on Intrinsic Heat Tolerance in Relation to Antioxidant Systems in Maize Seedlings, Aust.J. Plant Physiol., 1997, vol. 150, pp. 615–621.
Berridge, M.J., Inositol Trisphosphate and Calcium Signaling, Nature, 1993, vol. 361, pp. 315–325.
Clapham, D.E., Calcium Signaling, Cell, 1995, vol. 80, pp. 259–268.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, M., Wang, G.X. & Lin, J.S. Calcium Stimulates the Adaptation of Cultured Liquorice Cells to PEG-Induced Water Stress. Russian Journal of Plant Physiology 51, 518–524 (2004). https://doi.org/10.1023/B:RUPP.0000035746.98168.c6
Issue Date:
DOI: https://doi.org/10.1023/B:RUPP.0000035746.98168.c6