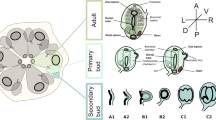
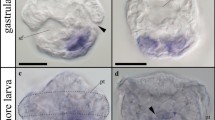
Abstract
Development of the muscle system in invertebrates (on the example of nematode Caenorhabditis elegans and Drosophila) and vertebrates (fish, birds, and mammals) demonstrates a number of common patterns at the level of molecular and genetic control mechanisms but also a number of distinctive features. C. elegans muscle is formed from several cells of different origin at the earliest developmental stages, while inductive interactions play a critical role in Drosophila: the mesoderm, which is a source of muscle formation, receives the inductive signals from the neighboring ectoderm as expression of Dpp and Hhgenes. In vertebrates, the induction and commitment of cells to the myogenic lineage are promoted by morphogenetic signals from the neighboring notochord (as expression of Shh gene) and neural tube (as expression of Wnt and Shh genes). The inductive signals entering the mesoderm are related to the subsequent activation of genes encoding protein transcription factors of bHLH gene family and analogs. These include hlh-1 and CeTwi in C. elegans; Twi and nau in Drosophila; and MyoD, Myf5, myogenin, and MRF4 in vertebrates. The diversity of myosin isoforms in different animals is provided by either gene duplication or alternative splicing of particular genes.
Similar content being viewed by others
REFERENCES
Araki, I., Saiga, H., Makabe, K., and Satoh, N., Expression of Amd1, a Gene for a MyoD1-Related Factor in the Ascidian Halocynthia roretzi, Roux's Arch. Dev. Biol., 1994, vol. 203, pp. 320–327.
Arnold, H.H. and Siddiqui, M., Control of Embryonic Development: Isolation and Proliferation of Chick Heart Myosin Light Chain mRNA and Quantitation with a cDNA Probe, Biochemistry, 1979, vol. 18, p. 647.
Azpiazu, N. and Frasch, M., Tinman and Bagpipe: Two Homeo Box Genes That Determine Cell Fates in the Dorsal Mesoderm of Drosophila, Genes Devel., 1993, vol. 7, pp. 1325–1340.
Azpiazu, N., Lawrence, P., Vincent, J.P., and Frasch, M., Segmentation and Specification of the Drosophila Mesoderm, Genes Devel., 1996, vol. 10, pp. 3183–3194.
Bandman, E., The Avian Myosin Heavy Chains, Gene Expression in Neuromuscular DevelopmentBlau, H. and Kelly, A.M.}, Eds., New York: Raven, 1992, pp. 68–82.
Bandman, E., Matsuda, R., and Strihman, R.C., Developmental Appearance of Myosin Heavy and Light Chain Isoforms in vivo Chicken Skeletal Muscle, Dev. Biol., 1982, vol. 93, p. 508.
Barton, P. and Buckingham, M.E., The Myosin Alkali Light Chain Proteins and Their Genes, Biochem. J.1985}, vol. 231, pp. 249–257.
Barton, P., Robert, B., Fiszman, M.Y., et al., The Same Myosin Alkali Light Chain Is Expressed in Adult Cardiac Atria and in Fetal Skeletal Muscle, J. Muscle Res. Cell Motil., 1985, vol. 6, p. 461.
Bate, M., The Embryonic Development of Larval Muscles in Drosophila, Development (Cambridge, UK), 1990, vol. 110, pp. 791–804.
Baylies, M.K. and Bate, M., Twist, a Myogenic Switch in Drosophila, Science, 1996, vol. 272, pp. 1481–1484.
Baylies, M.K. and Michelson, A.M., Invertebrate Myogenesis: Looking for Back to the Future of Muscle Development, Curr. Opin. Gen. Devel., 2001, vol. 11, pp.431–439.
Baylies, M.K., Bate, M., and Ruiz-Gomez, M., Myogenesis, a View from Drosophila, Cell (Cambridge, Mass.), 1998, vol. 93, pp. 921–927.
Benfield, P.A., Lowey, S., Le Blanc, D.D., and Waller, G.S., Fractionation of Myosin Isozymes from Adult and Embryonic Chicken Pectoralis Muscle by Immunoaffinity Chromatography, J. Muscle Res. Cell Motil., 1983, vol. 4, pp. 69--698.
Bernstein, S.I., Hansen, C.J., Becker, K.D., et al., Alternative RNA Splicing Generates Transcripts Encoding a Thorax-Specific Isoform of Drosophila melanogaster Myosin Heavy Chain, Mol. Cell. Biol., 1986, vol. 6, pp. 2511–2519.
Bodmer, R., The Gene tinman Is Required for Specification of the Heart and Visceral Muscles in Drosophila, Development (Cambridge, UK), 1993, vol. 118, pp. 719–729.
Bour, B.A., O'Brian, M.A., Lockwood, W.L., et al., Drosophila MEF2, a Transcription Factor That Is Essential for Myogenesis, Genes Devel., 1995, vol. 9, pp. 730–741.
Braun, T., Buschhausen, D.C., Bober, E., et al., A Novel Human Muscle Factor Related to but Distinct from MyoD1 Induced Myogenic Conversion in 10T1/2 Fibroblasts, EMBO J., 1989, vol. 8, pp. 701–710.
Braun, T., Bober, E., Winter, B., et al., Myf-6, a New Member of the Human Gene Family of Myogenic Determination Factors: Evidence for a Gene Cluster on Chromosome 12, EMBO J., 1990, vol. 9, pp. 821–829.
Breitbart, R.E., Nguyen, H.T., Medford, R.M., et al., Intricate Combinatorial Patterns of Exon Splicing Generate Multiple Regulation Troponin T Isoforms from a Single Gene, Cell (Cambridge, Mass.), 1985, vol. 41, pp. 67–82.
Castanon, I., Von Stetina, S., Kass, J., and Baylies, M.K., Dimerization Partners Determine the Activity of the Twist BHLH Protein during Drosophila Mesoderm Development, Development (Cambridge, UK), 2001, vol. 128, pp. 3145–3159.
Chen, L., Krause, M., Sepanski, M., and Fire, A., The Caenorhabditis elegans MYOD Homologue HLH-1 Is Essential for Proper Muscle Function and Complete Morphogenesis, Development, 1994, vol. 120, pp. 1631–1641.
Collier, V.I., Kronert, W.A., O'Donnell, P.T., et al., Alternative Myosin Hinge Regions Are Utilized in a Tissue-Specific Fashion That Correlates with Muscle Contraction Speed, Genes Devel., 1990, vol. 4, pp. 885–895.
Crow, M.T., Olson, P.S., and Stockdale, F.E., Myosin Light Chain Expression during Avian Muscle Development, J. Cell Biol., 1983, vol. 96, pp. 736–744.
Cummins, C. and Anderson, P., Regulatory Myosin LightChain Genes of Caenorhabditis elegans, Mol. Cell. Biol. 1988, vol. 8, pp. 5339–5349.
Davis, R.L., Weintraub, H., and Lassar, A.B., Expression of a Single Transfected cDNA Converts Fibroblasts to Myoblasts, Cell (Cambridge, Mass.), 1987, vol. 51, pp. 987–1000.
Dunin-Borokowski, O., Brown, N., and Bate, M., Anterior-Posterior Subdivision and the Diversification of the Mesoderm in Drosophila, Development (Cambridge, UK), 1995, vol. 121, pp. 4183–4193.
Edmonson, D.G. and Olson, E.N., A Gene with Homology to the myc Similarity Region of MyoD1 Is Expressed during Myogenesis and Is Sufficient to Activate the Muscle Differentiation Program, Genes Devel., 1989, vol. 3, pp. 628–640.
Epstein, H.F. and Bernstein, S.I., Genetic Approaches to Understanding Muscle Development, Dev. Biol.1992}, vol. 154, pp. 231–244.
Epstein, H.F., Ortiz, I., and Mackinnon, L.A.T., The Alteration of Myosin Isoform Compartmentation in Specific Mutants of Caenorhabditis elegans, J. Cell Biol., 1986, vol. 103, pp. 985–993.
Focant, B., Huriaux, F., Vandewalle, P., et al., Myosin, Parvalbumin and Myofibril Expression in Barbel (Barbus barbus L.) Lateral White Muscle during Development, Fish Physiol. Biochem., 1992, vol. 10, pp. 133–143.
Frank, D. and Harland, R., Transient Expression of XMyoD in Nonsomatic Mesoderm of Xenopus Gastrulae, Development (Cambridge, UK), 1991, vol. 113, pp. 1387–1393.
Frank, D. and Weeds, A.G., The Amino-Acid Sequences of the Alkali Light Chains of Rabbit Skeletal-Muscle Myosin, Eur. J. Biochem., 1974, vol. 44, pp. 317–334.
Frasch, M., Induction of Visceral and Cardiac Mesoderm by Ectodermal Dpp in the Early Drosophila Embryo, Nature (London), 1995, vol. 374, pp. 464–467.
Gautier, G.F. and Lowey, S., Polymorphism of Myosin among Skeletal Muscle Fibers, J. Cell Biol., 1977, vol. 74, pp. 760–779.
Gautier, G.F. and Lowey, S., Distribution of Myosin Isozymes among Skeletal Muscle Fiber Types, J. Cell Biol., 1979, vol. 81, pp. 10–25.
George, E.L., Ober, M.B., and Emerson, C.P., Jr., Functional Domains of the Drosophila melanogaster Muscle Myosin Heavy-Chain Gene Are Encoded by Alternatively Spliced Exons, Mol. Cell. Biol., 1989, vol. 9, pp. 2957–2974.
Harfe, B.D., Gomes, A.V., Keyon, C., et al., Analysis of a Caenorhabditis elegans Twist Homolog Identifies Conserved and Divergent Aspects of Mesodermal Patterns, Genes Devel., 1998, vol. 12, pp. 2623–2635.
Harvey, R.P., MyoD Protein Expression in Xenopus Embryos Closely Follows a Mesoderm Induction-Dependent Amplification of MyoD Transcription and Is Synchronous across the Future Somite Axis, Mech. Devel., 1992, vol. 37, pp. 14---47.
Hollenberg, S.M., Cheng, P.F., and Weintraub, H., Use of a Conditional MyoD Trans-Activation and Muscle Determination, Proc. Natl. Acad. Sci. USA, 1993, vol. 90, pp. 802--8032.
Hresko, M.C., Williams, B.D., and Waterston, R.H., Assembly of Body Wall Muscle and Muscle Cell Attachment Structures in Caenorhabditis elegans, J. Cell Biol.1994}, vol. 124, pp. 491–506.
Huriaux, F. and Focant, B., Isolation and Characterization of the Three Light Chains from Carp White Muscle Myosin, Arch. Int. Physiol. Biochim., 1977, vol. 85, pp. 917–929.
Irving, T., Bhattacharya, S., Tesic, I., et al., Changes in Myofibrillar Structure and Function Produced by N-Terminal Deletion of the Regulatory Light Chain in Drosophila, J. Muscle Res. Cell Motil., 2001, vol. 22, pp. 675–683.
Krause, M., Fire, A., White-Harrison, S., et al., CeMyoD Accumulation Defines the Body Wall Muscle Cell Fate during C. elegans Embryogenesis, Cell (Cambridge, Mass.), 1990, vol. 63, pp. 907–919.
Kumar, S., Cribbs, L., Delaney, P., et al., Heart Myosin Light Chain 2 Gene. Nucleotide Sequence of Full Length cDNA and Expression in Normal and Hypertensive Rat, J. Biol. Chem., 1986, vol. 261, pp. 2866–2874.
Lilly, B., Zhao, B., Ranganyakulu, G., et al., Requirement of MADS Domain Transcription Factor D-MEF2 For Muscle Formation in Drosophila, Science, 1995, vol. 267, pp. 68--693.
Lowey, S., Benfiels, P.A., Le Blanc, D.D., et al., Characterization of Myosin from Embryonic and Development: Molecular and Cellular Control, New York: Cold Spring Har-bor Lab., 1982, pp. 15–24.
Lyons, G.E., Haselgrove, J., Kelly, A.M., et al., Myosin Transitions in Developing Fast and Slow Muscles of the Cat Hind Limb, Differentiation (Berlin), 1983, vol. 25, 168–175.
Mahdavi, V., Chambers, A.P., and Nadal-Ginard, B., Cardiac and Myosin Heavy Chain Genes Are Organized in Tandem, Proc. Natl. Acad. Sci. USA, 1984, vol. 81, pp. 2626–2630.
Maroto, M., Reshef, R., Münsterberg, A.E., et al., Ectopic Pax-3 Activates MyoD and Myf-5 Expression in Embryonic Mesoderm and Neural Tissue, Cell (Cambridge, Mass.), 1997, vol. 89, pp. 139–148.
Masaki, T., Immunochemical Comparison of Myosins from Chicken Cardiac, Fast White, Slow Red, and Smooth Muscle, J. Biochem. (Tokyo), 1974, vol. 76, pp. 441–448.
Matsuda, R., Maita, T., Kato, Y., et al., Amino Acid Sequence of the Cardiac L-2A, L-2B and Gizzard 17 000 Light Chains of Chicken Muscle Myosin, FEBS Lett., 1981, vol. 135, 23--236.
Michaelson, A.M., Abmayr, S.M., Bate, M., et al., Expression of a MyoD Family Member Prefigures Muscle Pattern in Drosophila Embryos, Genes Devel., 1990, vol. 4, pp. 2086–2097.
Miller, D.M., I, II., Ortiz, I., Berliner, G.C., and Epstein, H.F., Differential Localization of Two Myosins within Nematode Thick Filaments, Cell (Cambridge, Mass.), 1983, vol. 34, pp. 477–490.
Münsterberg, A.E. and Lassar, A.B., Combinatorial Signals from the Neural Tube, Floor Plate and Notochord Induce Myogenic bHLH Gene Expression in the Somite, Development (Cambridge, UK), 1995, vol. 121, p. 651.
Münsterberg, A.E., Kitajewski, J., Bumcrot, D.A., et al., Combinatorial Signaling from Sonic hedgehog and Wnt Family Members Induces Myogenic bHLH Gene Expression in the Somite, Genes Devel.1995}, vol. 9, p. 2911.
Nabeshima, Y., Fujii-Kiriyama, Y., Muramatsu, M., et al., Alternative Transcription and Two Modes of Splicing Result in Two Myosin Light Chains from One Gene, Nature (Lon-don), 1985, vol. 908, pp. 333–338.
Nareiko, V.G., Myosin Isoforms in Developing Skeletal Muscle of Loach, Ontogenez (Moscow), 1988, vol. 19, pp. 601–606.
Nareiko, V.G. and Ozernyuk, N.D., Differences in Composition of Contractile Proteins between Red and While Skeletal Muscles in Teleostean Fish, Dokl. Akad. Nauk SSSR1988}, vol. 298, no. 4, pp. 1022–1024.
Nguen, H.T., Gubits, R.M., Wydro, R.M., et al., Sarcomeric Myosin Heavy Chain Is Coded by a Highly Conserved Multigene Family, Proc. Natl. Acad. Sci. USA, 1982, vol. 79, 5230–5234.
Obinata, T., Masaki, T., and Takano, H., Types of Myosin Light Chains Present during Development of Fast Skeletal Muscle in Chick Embryos, J. Biochem. (Tokyo), 1980, vol. 87, pp. 81–88.
Olson, E.N., Interplay between Proliferation and Differentiation within the Myogenic Lineage, Dev. Biol., 1992, vol. 154, pp. 261–268.
Olson, E.N. and Klein, W.H., bHLH Factors in Muscle Development: Dead Lines and Commitments, What to Leave in and What to Leave Out, Genes Devel., 1994, vol. 8, pp. 1–8.
Ontell, M., Ontell, M.P., and Buckingham, M., Muscle-Specific Gene Expression during Myogenesis in the Mouse, Microscopy Res. Tech., 1995, vol. 30, pp. 354–361.
Ozernyuk, N.D., Regulation of Myogenesis, Izv. Ross. Akad. Nauk, Ser. Biol.1998}, no. 3, pp. 330–343.
Ozernyuk, N.D., Smirnova, Yu.A., Nareiko, V.G., and Zinov'eva, R.D., Specific Features of Skeletal Muscle Differentiation in Fish: Molecular Biology Approaches, Izv. Ross. Akad. Nauk, Ser. Biol., 2004, no. 3, pp. 261–268.
Paterson, B.M., Walldorf, U., Eldridge, J., et al., The Drosophila Homologue of Vertebrate Myogenic-Determination Genes Encodes a Transiently Expressed Nuclear Protein Marking Primary Myogenic Cells, Proc. Natl. Acad. Sci. USA, 1991, vol. 88, pp. 3782–3786.
Pownal, M.E. and Emerson, C.P., Sequential Activation of Three Myogenic Regulatory Genes during Somite Morpho-genesis in Quail Embryos, Dev. Biol., 1992, vol. 151, p. 67.
Rawls, A. and Olson, E., MyoD Meets Its Marker, Cell, 1997, vol. 89, pp. 5–8.
Rhodes, S.J. and Kenieszny, S.F., Identification of MRF4: A New Member of the Muscle Regulatory Factor Gene Family, Genes Devel., 1989, vol. 3, pp. 2050–2056.
Riechmann, V., Irion, U., Wilson, R., et al., Control of Cell Fates and Segmentation in the Drosophila Mesoderm, Development (Cambridge, UK), 1997, vol. 124, pp. 2915–2922.
Robert, B., Daubas, P., Akimenko, M.-A., et al., A Single Locus in the Mouse Encodes Both Myosin Light Chains 1 And 3, a Second Locus Corresponds to a Related Pseudogene, Cell (Cambridge, Mass.), 1984, vol. 39, pp. 129–140.
Rong, P.M., Teillet, M.A., Ziller, C., et al., The Neural Tube/Notochord Complex Is Necessary for Vertebral but Not Limb and Body Wall Striated Muscle Differentiation, Development, 1992, vol. 115, pp. 657–672.
Rozek, C.E. and Davidson, N., Differential Processing of RNA Transcribed from Single-Copy Drosophila Myosin Heavy Chain Gene Produces Four mRNAs That Encode Two Polypeptides, Proc. Natl. Acad. Sci. USA1986}, vol. 83, pp. 2128–2132.
Ruiz-Gomez, M., Coutts, N., Price, A., et al., Drosophila Dumbfounded: A Myoblast Attractant Essential for Fusion, Cell (Cambridge, Mass.), vol. 102, pp. 189–198.
Rushbrook, J.A. and Stracher, A., Comparison of Adult, Embryonic and Dystrophic Myosin Heavy Chain from Chicken Muscle by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis and Peptide Mapping, Proc. Natl. Acad. Sci. USA, 1979, vol. 76, pp. 4331–4334.
Sreter, F.A., Balint, M., and Gergely, J., Structural and Functional Changes of Myosin during Development. Comparison with Adult Fast, Slow and Cardiac Myosin, Dev. Biol., 1975, vol. 46, pp. 317–325.
Staehling-Hampton, K., Hoffman, F., Baylies, M., et al., Dpp Induces Mesodermal Gene Expression on Drosophila, Nature (London), 1994, vol. 372, pp. 783–786.
Sulston, J.E. and Horwitz, H.R., Post-Embryonic Cell Lineages of the Nematode Caenorhabditis elegans, Dev. Biol., 1977, vol. 56, pp. 110–156.
Sulston, J.E., Schierenberg, E., White, J.G., and Thomson, J.N., The Embryonic Cell Lineage of the Nematode Caenorhabditis elegans, Dev. Biol.1983}, vol. 100, pp. 64–119.
Tajbakhsh, S., Rocancourt, D., Cossu, G., and Buckingham, M., Redefining the Genetic Hierachies Controlling Skeletal Myogenesis: Pax-3 and Myf-5 Act Upstream of MyoD, Cell (Cambridge, Mass.), 1997, vol. 89, pp. 127–138.
Takano-Ohmuro, H., Takahashi, S., Hirose, G., and Maruyama, K., Phosphorylated and Dephosphorylated Myosin Light Chains of Drosophila Fly and Larva, Comp. Biochem. Physiol., B: Comp. Biochem., 1990, vol. 95, pp. 171–177.
Taylor, M.V., Beatty, K.E., Hunter, H.K., and Baylies, M.K., Drosophila MEF2 Is Regulated by Twist and Is Expressed in Both the Primordia and Differentiated Cells of the Embryonic Somatic, Visceral and Heart Musculature, Mech. Devel., 1995, vol. 50, pp. 29–41.
Teillet, M.A. and LeDouarin, N.M., Consequences of Neural Tube and Notochord Excision on the Development of the Peripheral Nervous System in the Chick Embryos, Dev. Biol., 1983, vol. 98, pp. 192–211.
Venuti, J.M., Goldberg, L., Chakraborty, T., et al., A Myogenic Factor from Sea Urchin Embryos Capable of Programming Muscle Differentiation in Mammalian Cells, Proc. Natl. Acad. Sci. USA, 1991, vol. 88, p. 6219.
Weeds, A.G. and Lowey, S., Substructure of the Myosin Molecule. 2. The Light Chains of Myosin, J. Mol. Biol., 1971, vol. 61, pp. 701–725.
Weinberg, E.S., Allende, M.L., Kelly, C.S., et al., Developmental Regulation of Zebrafish MyoD in Wild-Type, no tail and spadetail, Development (Cambridge, UK), 1996, vol. 122, pp. 271–280.
Whalen, R.G., Butler-Browne, G.S., and Gros, F., Identification of a Novel Form of Myosin Light Chain Present in Embryonic Muscle Tissue and Cultured Muscle Cells, J. Mol. Biol., 1978, vol. 126, pp. 415–431.
Whalen, R.G., Sell, S.M., Butler-Browne, G.S., et al., Three Myosin Heavy Chain Isoenzymes Appear Sequentially in Rat Muscle Development, Nature (London), 1981, vol. 292, pp. 805–809.
Winkelmann, D.A., Lowey, S., and Press, J.L., Monoclonal Antibodies Localize Changes on Myosin Heavy Chains Isozymes during Avian Myogenesis, Cell (Cambridge, Mass.), 1983, vol. 34, pp. 295–306.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ozernyuk, N.D. Comparative Properties of Myogenesis in Invertebrates and in Lower and Higher Vertebrates. Russian Journal of Developmental Biology 35, 360–369 (2004). https://doi.org/10.1023/B:RUDO.0000049609.55387.dc
Issue Date:
DOI: https://doi.org/10.1023/B:RUDO.0000049609.55387.dc