Abstract
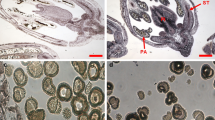
We investigated changes in gene expression in Iris hollandicaflowers by microarray technology. Flag tepals were sampled daily, from three days prior to flower opening to the onset of visible senescence symptoms. Gene expression profiles were compared with biochemical data including lipid and protein degradation and DNA coiling, and with morphological data. Plasmodesmata of mesophyll cells closed about two days before flower opening, while in the epidermis they closed concomitant with opening. Similarly, the onset of visible senescence in the epidermis cells occurred about two days later than in the mesophyll. About 1400 PCR-amplified clones, derived from a subtractive cDNA library enriched for tepal-specific genes, were spotted and about 240 clones, including 200 that were expressed most differentially, were sequenced. The expression patterns showed three main clusters. One exhibited high expression during tepal growth (cluster A). These genes were putatively associated with pigmentation, cell wall synthesis and metabolism of lipids and proteins. The second cluster (B) was highly expressed during flower opening. The third cluster (C) related to the final stages of senescence, with genes putatively involved in signal transduction, and the remobilization of phospholipids, proteins, and cell wall compounds. Throughout the sampling period, numerous plant defence genes were highly expressed. We identified an ion channel protein putatively involved in senescence, and some putative regulators of transcription and translation, including a MADS-domain factor.
Similar content being viewed by others
References
Abraham, K.J., Pierce, M.L. and Essenberg, M. 1999. The phytoalexins desoxyhemigossypol and hemigossypol are elicited by Xanthomonas in Gossypium cotyledons. Phytochemistry 52: 829–836.
Ahmed, K., Gerber, D.A. and Cochet, C. 2002. Joining the cell survival squad: an emerging role of protein kinase CK2. Trends Cell Biol. 12: 226–230.
Altschul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W. and Lipman, D.J. 1997. Gapped BLAST and PSIBLAST: a new generation of protein database search programs. Nucl. Acids Res. 25: 3389–3402.
Arazi, T., Kaplan, B. and Fromm, H. 2000. A high-affinity calmodulin-binding site in a tobacco plasma-membrane channel protein coincides with a characteristic element of cyclic nucleotide-binding domains. Plant Mol. Biol. 42: 591–601.
Bailly, C., Corbineau, F. and van Doorn, W.G. 2000. Free radical scavenging and senescence in Iris tepals. Plant Physiol. Biochem. 39: 649–656.
Baker, J.E. and Takeo, T. 1973. Acid phosphatase in plant tissues. Changes in activity and multiple forms in tea leaves and tomato fruit during maturation and senescence. Plant Cell Physiol. 14: 459–471.
Bhalerao, R., Keskitalo, J., Sterky, F., Erlandsson, R., Bjorkbacka, H., Birve, S.J., Karlsson J., Gardestrom, P., Gustafsson, P., Lundeberg, J. and Jansson, S. 2003. Gene expression in autumn leaves. Plant Physiol. 131: 430–442.
Bieleski, R. 1995. Onset of phloem export from senescent tepals of daylily. Plant Physiol. 109: 557–565.
Blein, J.P., Coutos-Thévenot, P., Marion, D. and Ponchet, M. 2002. From elicitins to lipid-transfer proteins: a new insight in cell signalling involved in plant defence mechanisms. Trends Plant Sci. 7: 293–296.
Borg-Karlson, A.K., Valterova, I. and Nilsson, L.A. 1994. Volatile compounds from flowers of six species in the family Apiaceae: bouquets for different pollinators? Phytochemistry 35: 111–119.
Bouvier, F., Suire C., d'Harlingue, A., Backhaus, R.A. and Camara, B. 2000. Molecular cloning of geranyl diphosphate synthase and compartmentation of monoterpene synthesis in plant cells. Plant J. 24: 241–252.
Bradford, M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein using the principle of protein-dye binding. Ann. Biochem. 72: 248–254.
Buchanan-Wollaston, V. 1997. The molecular biology of leaf senescence. J. Exp. Bot. 48: 181–199.
Carzaniga, R., Sinclair, L., Fordham-Skelton, A.P., Harris, N. and Croy, R.R.D. 1994. Cellular and subcellular distribution of saporins, type-1 ribosome-inactivating proteins, in soapwort (Saponaria officinalis L.). Planta 194: 461–470.
Celikel, F.G. and van Doorn, W.G. 1995. Solute leakage, lipid peroxidation, and protein degradation during the senescence of Iris tepals. Physiol. Plant 94: 515–524.
Chang, S., Puryear, J. and Cairney, J. 1993. A simple and efficient method to isolate RNA from pine trees. Plant Mol. Biol. Rep. 11: 114–117.
Chang, M.S., Chang, C.L., Huang, C.J. and Yang, Y.C. 2000. p29, a novel GCIP-interacting protein, localizes in the nucleus. Biochem. Biophys. Res. Comm. 279: 732–737.
Crawford, K.M. and Zambryski, P.C. 2001. Non-targeted and targeted protein movement through plasmodesmata in leaves in different developmental and physiological states. Plant Physiol. 125: 1802–1812.
De Bodt, S., Raes, J., Van de Peer, Y. and Theissen G. 2003. And there were many: MADS goes genomic. Trends Plant Sci. 8: 475–483.
De Moliner, E., Moro, S., Sarno, S., Zagotto, G., Zanotti, G., Pinna, L.A. and Battistutta, R. 2003. Inhibition of protein kinase CK2 by anthraquinone-related compounds. A structural insight. J. Biol. Chem. 278: 1831–1836.
Eason, J.R., Johnston, J.W., de Vre, L., Sinclair, B.K. and King, G.A. 2000. Amino acid metabolism in senescing Sandersonia aurantiaca flowers: cloning and characterization of asparagine synthetase and glutamine synthetase cDNAs. Aust. J. Plant Physiol. 27: 389–396.
Fernandez, D.E., Heck, G.R., Perry, S.E., Patterson, S.E., Bleecker, A.B. and Fang, S.C. 2000. The embryo MADS domain factor AGL15 acts postembryonically: inhibition of perianth senescence and abscission via constitutive expression. Plant Cell 12: 183–197.
Fernandez, H.A., Kallenbach, K., Seghezzi, G., Grossi, E., Colvin, S., Schneider, R., Mignatti, P. and Galloway, A. 1999. Inhibition of endothelial cell migration by gene transfer of tissue inhibitor of metalloproteinases-1. J. Surg. Res. 82: 156–162.
Fiebig, A., Mayfield, J.A., Miley, N.L., Chau, S., Fischer, R.L. and Preuss, D. 2000. Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell 12: 2001–2008.
Fiucci, G., Lespagnol, A., Stumptner-Cuvelette, P., Beaucourt, S., Duflaut, D., Susini, L., Amson, R. and Telerman, A. 2003. Genomic organization and expression of mouse Tpt1 gene. Genomics 81: 570–578.
Graham, I.A., Denby, K.J. and Leaver, C.J. 1994. Carbon catabolite repression regulates glyoxylate cycle gene expression in cucumber. Plant Cell 6: 761–772.
Halperin, T., Zheng, B., Itzhaki, H., Clarke, A.K. and Adam, Z. 2001. Plant mitochondria contain proteolytic and regulatory subunits of the ATP-dependent Clp protease. Plant Mol. Biol. 45: 461–468.
Hidalgo, P., Garreton, V., Berrios, C.G., Ojeda, H., Jordana, X. and Holuigue, L. 2001. A nuclear casein kinase 2 activity is involved in early events of transcriptional activation induced by salicylic acid in tobacco. Plant Physiol. 125: 396–405.
Jang, S., Hong, M.Y., Chung, Y.Y. and An, G. 1999. Ectopic expression of tobacco MADS genes modulates flowering time and plant architecture. Mol. Cells 9: 576–586.
Jones, M.L., Larsen, P.B. and Woodson, W.R. 1995. Ethyleneregulated expression of a carnation cysteine proteinase during flower petal senescence. Plant Mol. Biol. 28: 505–512.
Khan, M.B. and Harborne, J.B. 1990. Induced alkaloid defence in Atropa acuminata in response to mechanical and herbivore damage. Chemoecology 1: 77–80.
Köhler, C., Merkle, T., Roby, D. and Neuhaus, G. 2001. Developmentally regulated expression of a cyclic nucleotide-gated ion channel from Arabidopsis indicates its involvement in programmed cell death. Planta 213: 327–332.
Kotilainen, M., Helariutta, Y., Elomaa, P., Paulin, L. and Teeri, T.H. 1994. A corolla-and carpel-abundant, non-specific lipid transfer protein gene is expressed in the epidermis and parenchyma of Gerbera hybrida var. Regina (Compositae). Plant Mol. Biol. 26: 971–978.
Lee, R.H. and Chen, S.C.G. 2002. Programmed cell death during rice leaf senescence is nonapoptotic. New Phytol. 155: 25–32.
Liou, J.Y., Krishnan, P., Hsieh, C.C., Dutschman, G.E. and Cheng, Y.C. 2003. Assessment of the effect of phosphorylated metabolites of anti-human immunodeficiency virus and antihepatitis B virus pyrimidine analogs on the behavior of human deoxycytidylate deaminase. Mol. Pharmacol. 63: 105–110.
Lu, Z.X., Wu, M., Loh, C.S., Yeong, C.Y. and Goh, C.J. 1993. Nucleotide sequence of a flower-specific MADS box cDNA clone from orchid. Plant Mol. Biol. 23: 901–904.
Lucas, W.J., Ding, B. and van der Schoot, C. 1993. Plasmodesmata and the supracellular nature of plants. New Phytol. 125: 435–476.
Matile, P. 1997. The vacuole and cell senescence. In: R.A. Leigh, D. Sanders and J.A. Callow (Eds.) The Plant Vacuole. Advances in Botanical Research Vol. 25, Academic Press, San Diego, CA, pp. 87–112.
Matile, P. and Winkenbach, F. 1971. Function of lysosomes and lysosomal enzymes in the senescing corolla of the morning glory (Ipomoea purpurea). J. Exp. Bot. 22: 759–771.
Morris, K., Mackerness, S.A.H., Page, T., John, C.F., Murphy, A.M., Carr, J.P. and Buchanan-Wollaston, V. 2000. Salicylic acid has a role in regulating gene expression during leaf senescence. Plant J. 23: 677–685.
Munnik, T., Arisz, S.A., de Vrije, T. and Musgrave, A. 1995. G protein activation stimulates phospholipase D signaling in plants. Plant Cell 7: 2197–2210.
O'Brien, I.E.W., Baguley, B.C., Murray, B.G., Morris, B.A.M. and Ferguson, I.B. 1998. Early stages of the apoptotic pathway in plant cells are reversible. Plant J. 13: 803–814.
Ormenese, S., Havelange, A., Bernier, A. and van der Schoot, C. 2002. The shoot apical meristem of Sinapis alba L. expands its central symplasmic field during the floral transition. Planta 215: 67–78.
Ouvrard, O., Cellier, F., Ferrare, K., Tousch, D., Lamaze, T., Dupuis, J.M. and Casse-Delbart, F. 1996. Identification and expression of water stress-and abscisic acid-regulated genes in a drought-tolerant sunflower genotype. Plant Mol. Biol. 31: 819–829.
Page, T., Griffiths, G. and Buchanan-Wollaston, V. 2001. Molecular and biochemical characterization of postharvest senescence in broccoli. Plant Physiol. 125: 718–727.
Pariasca, J.A.T., Sunaga, A., Miyazaki, T., Hisaka, H., Sonoda, M., Nakagawa, H. and Sato, T. 2001. Cloning of cDNAs encoding senescence-associated genes, ACC synthase and ACC oxidase from stored snow pea pods (Pisum sativum L. var. saccharatum). and their expression during pod storage. Postharvest Biol. Technol. 22: 239–247.
Punja, Z.K. 2001. Genetic engineering of plants to enhance resistance to fungal pathogens: a review of progress and future prospects. Can. J. Plant Pathol. 23: 216–235.
Quirino, B.F., Noh, YS., Himelblau, E. and Amasino, R.M. 2000. Molecular aspects of leaf senescence. Trends Plant Sci. 5: 278–282.
Rinne, P.L.H. and van der Schoot, C. 1998. Symplasmic fields in the tunica of the shoot apical meristem coordinate morphogenetic events. Development 125: 1477–1485.
Ross, J.R., Hee, N.K., D'Auria, J.C. and Pichersky, E. 1999. S-Adenosyl-L-methionine:salicylic acid carboxyl methyltransferase, an enzyme involved in floral scent production and plant defense, represents a new class of plant methyltransferases. Arch. Biochem. Biophys. 367: 9–16.
Sessions, A. Yanofsky, M.F. and Weigel, D. 2000. Cell-cell signalling and movement by the floral transcription factors LEAFY and APETALA1. Science 289: 779–781.
Smith, M.T., Saks, Y. and van Staden, J. 1992. Ultrastructural changes in the petals of senescing flowers of Dianthus caryophyllus. Ann. Bot. 69: 277–285.
Turner, G., Gershenzon, J., Nielson, E.E., Froehlich, J.E. and Croteau, R. 1999. Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiol. 120: 879–886.
Van Damme, E.J.M., Barre, A., Barbieri, L., Valbonesi, P., Rouge, P., Van Leuven, F., Stirpe, F. and Peumans, W.J. 1997. Type 1 ribosome-inactivating proteins are the most abundant proteins in iris (Iris hollandica var. Professor Blaauw) bulbs: characterization and molecular cloning. Biochem. J. 324: 963–970.
van Doorn, W.G. 2001. Categories of petal senescence and abscission: a re-evaluation. Ann. Bot. 87: 447–456.
van Poecke, R.M.P., Posthumus, M.A. and Dicke, M. 2001. Herbivore-induced volatile production by Arabidopsis thaliana leads to attraction of the parasitoid Cotesia rubecula: chemical, behavioral, and gene-expression analysis. J. Chem. Ecol. 27: 1911–1928.
Vrebalov, J., Ruezinsky, D., Padmanabhan, V., White, R., Medrano, D., Drake, R., Schuch, W. and Giovannoni, J. 2002. A MADSbox gene necessary for fruit ripening at the tomato ripeninginhibitor (rin) locus. Science 296: 343–346.
Wagstaff, C., Leverentz, M.K., Griffiths, G., Thomas, B., Chanasut, U., Stead, A.D. and Rogers, H.J. 2002. Cysteine protease gene expression and proteolytic activity during senescence of Alstroemeria petals. J. Exp. Bot 53: 233–240.
Walker, K. and Croteau, R. 2001. Taxol biosynthetic genes. Phytochemistry 58: 1–7.
Wiemken, V., Wiemken, A. and Matile, P. 1976. Physiologie der Blüten von Ipomoea tricolor (Cav.): Untersuchungen an abgeschnittenen Blüten und Gewinnung eines Phloemexsudates. Biochem. Physiol. Pflanzen 169: 363–376.
Winkenbach, F. 1970a. Zum Stoffwechsel der aufblühenden und welkenden Korolle der Prunkwinde Ipomoea purpurea. I. Beziehungen zwischen Gestaltwandel, Stofftransport, Atmung und Invertaseaktivität. Ber. Schweiz. Bot. Gesellsch. 80: 374–390.
Winkenbach, F. 1970b. Zum Stoffwechsel der aufblühenden und welkenden Korolle der Prunkwinde Ipomoea purpurea. II. Funktion und de novo Synthese lysosomaler Enzyme beim Welken. Ber. Schweiz. Bot. Gesellsch. 80: 391–406.
Wyllie, S.G. and Fellman, J.K. 2000. Formation of volatile branched chain esters in bananas (Musa sapientum L.). J. Agric. Food Chem. 48: 3493–3496.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Doorn, W., Balk, P., van Houwelingen, A. et al. Gene expression during anthesis and senescence in Iris flowers. Plant Mol Biol 53, 845–863 (2003). https://doi.org/10.1023/B:PLAN.0000023670.61059.1d
Issue Date:
DOI: https://doi.org/10.1023/B:PLAN.0000023670.61059.1d