Abstract
Purpose. To investigate the impact of lipidic formulation type on in vitro dispersion and digestion properties and the relationship to oral bioavailability, using danazol as a model lipophilic poorly water-soluble drug.
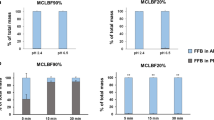
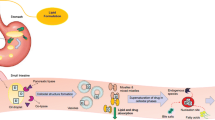
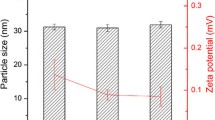
Methods. Three lipid-based danazol formulations [a long-chain triglyceride solution (LCT-solution) and self-microemulsifying drug delivery systems (SMEDDS) based on long-chain (C18) lipids (LC-SMEDDS) and medium-chain (C8-C10) lipids (MC-SMEDDS)] were adminis- tered to fasted beagle dogs and compared with a micronized danazol formulation administered postprandially and in the fasted state. In vitro dispersion and particle size data for the two SMEDDS were compared, and the distribution/solubilization patterns of danazol across the various phases produced during in vitro digestion quantified.
Results. The LCT-solution and LC-SMEDDS formulations significantly enhanced the oral bioavailability of danazol when compared to fasted administration of the powder formulation. In contrast, and despite displaying excellent dispersion properties, the MC-SMEDDS resulted in little enhancement in danazol bioavailability. In support of the in vivo findings, in vitro digestion of the medium-chain formulation resulted in significant drug precipitation when compared with the long-chain lipid formulations.
Conclusions. Digestion of microemulsion preconcentrate formulations based on medium-chain lipids may limit in vivo utility when compared with similar formulations based on long chain lipids.
Similar content being viewed by others
REFERENCES
A. J. Humberstone and W. N. Charman. Lipid-based vehicles for the oral delivery of poorly water soluble drugs. Adv. Drug Deliv. Rev. 25:103–128 (1997).
K. J. MacGregor, J. K. Embleton, J. E. Lacey, E. A. Perry, L. J. Solomon, H. Seager, and C. W. Pouton. Influence of lipolysis on drug absorption from the gastrointestinal tract. Adv. Drug Deliv. Rev. 25:33–46 (1997).
G. K. Kossena, B. J. Boyd, C. J. H. Porter, and W. N. Charman. Separation and characterisation of the colloidal phases produced on digestion of common formulation lipids and assessment of their impact on the apparent solubility of selected poorly water soluble drugs. J. Pharm. Sci. 92:634–648 (2003).
M. Lindström, H. Ljusberg-Wahren, K. Larsson, and B. Borgström. Aqueous lipid phases of relevance to intestinal fat digestion and absorption. Lipids 16:749–754 (1981).
P. P. Constantinides. Lipid microemulsions for improving drug dissolution and oral absorption: physical and biopharmaceutical aspects. Pharm. Res. 12:1561–1572 (1995).
T. Gershanik and S. Benita. Self-dispersing lipid formulations for improving oral absorption of lipophilic drugs. Eur. J. Pharm. Biopharm. 50:179–188 (2000).
R. M. Klauser, H. Irshik, J. Kletzmayr, I. Sturm, W. Brunner, W. Woloszczuk, and J. Kovarik. Pharmacokinetic cyclosporine A profiles under long-term Neoral treatment in renal transplant recipients: does fat intake still matter? Transplant. Proc. 29:3137–3140 (1997).
S. M. Khoo, A. J. Humberstone, C. J. H. Porter, G. A. Edwards, and W. N. Charman. Formulation design and bioavailability assessment of lipidic self-emulsifying formulations of halofantrine. Int. J. Pharm. 167:155–164 (1998).
D. J. Hauss, S. E. Fogal, J. V. Ficorilli, C. A. Price, T. Roy, A. A. Jayaraj, and J. J. Keirns. Lipid-based delivery systems for improving the bioavailability and lymphatic transport of a poorly watersoluble LTB4 inhibitor. J. Pharm. Sci. 87:164–169 (1998).
C. W. Pouton. Formulation of self-emulsifying drug delivery systems. Adv. Drug Deliv. Rev. 25:47–58 (1997).
A. M. Kaukonen, B. J. Boyd, C. J. H. Porter, and W. N. Charman. Drug solubilization behaviour during in vitrodigestion of simple triglyceride lipid solution formulations. Pharm. Res. 21: 245–253 (2004).
A. M. Kaukonen, B. J. Boyd, W. N. Charman, and C. J. H. Porter. Drug solubilization behaviour during in vitrodigestion of suspension formulations of poorly water-soluble drugs in triglyceride lipids. Pharm. Res. 21:254–260 (2004).
C. J. H. Porter, A. M. Kaukonen, A. Taillardat-Bertschinger, B. J. Boyd, J. M. O'Connor, G. A. Edwards, and W. N. Charman. Use of in vitrolipid digestion data to explain the in vivoperformance of triglyceride-based oral lipid formulations of poorly water-soluble drugs: studies with halofantrine. J. Pharm. Sci. 93: 1110–1121 (2004).
J. P. Reymond and H. Sucker. In vitromodel for ciclosporin intestinal absorption in lipid vehicles. Pharm. Res. 5:673–676 (1988).
A. Taillardat-Bertschinger, C. A. M. Martinet, P. A. Carrupt, M. Reist, G. Caron, R. Fruttero, and B. Testa. Molecular factors influencing retention on immobilized artifical membranes (IAM) compared to partitioning in liposomes and n-octanol. Pharm. Res. 19:729–737 (2002).
L. Sek, C. J. H. Porter, A. M. Kaukonen, and W. N. Charman. Evaluation of the in-vitro digestion profiles of long and medium chain glycerides and the phase behaviour of their lipolytic products. J. Pharm. Pharmacol. 54:29–41 (2002).
L. Sek, C. J. H. Porter, and W. N. Charman. Characterisation and quantification of medium chain and long chain triglycerides and their in vitro digestion products, by HPTLC coupled with in situdensitometric analysis. J. Pharm. Biomed. Anal. 25:651–661 (2001).
D. W. Hay, M. J. Cahalane, N. Timofeyeva, and M. C. Carey. Molecular species of lecithins in human gallbladder bile. J. Lipid Res. 34:759–768 (1993).
W. C. Duane, R. L. Ginsberg, and L. J. Bennion. Effects of fasting on bile acid metabolism and biliary lipid composition in man. J. Lipid Res. 17:211–219 (1976).
J. E. Staggers, O. Hernell, R. J. Stafford, and M. C. Carey. Physical-chemical behavior of dietary and biliary lipids during intestinal digestion and absorption. 1. Phase behavior and aggregation states of model lipid systems patterned after aqueous duodenal contents of healthy adult human beings. Biochemistry 29:2028–2040 (1990).
L. Erlich, D. Yu, D. A. Pallister, R. S. Levinson, D. G. Gole, P. A. Wilkinson, R. E. Erlich, L. E. Reeve, and T. X. Viegas. Relative bioavailability of danazol in dogs from liquid-filled hard gelatin capsules. Int. J. Pharm. 179:49–53 (1999).
22. M. C. Carey, D. M. Small, and C. M. Bliss. Lipid digestion and absorption. Annu. Rev. Physiol. 45:651–677 (1983).
J. E. Staggers, O. Hernell, R. J. Stafford, and M. C. Carey. Physical-chemical behaviour of dietary and biliary lipids during intestinal digestion and absorption. 1. Phase behaviour and aggregation states of model lipid systems patterned after aqueous duodenal contents of healthy adult human beings. Biochemistry 29: 2028–2040 (1990).
M. M. Nerurkar, P. S. Burton, and R. T. Borchardt. The use of surfactants to enhance the permeability of peptides through Caco-2 cells by inhibition of an apically polarized efflux system. Pharm. Res. 13:528–534 (1996).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Porter, C.J.H., Kaukonen, A.M., Boyd, B.J. et al. Susceptibility to Lipase-Mediated Digestion Reduces the Oral Bioavailability of Danazol After Administration as a Medium-Chain Lipid-Based Microemulsion Formulation. Pharm Res 21, 1405–1412 (2004). https://doi.org/10.1023/B:PHAM.0000036914.22132.cc
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000036914.22132.cc