Abstract
Purpose. To test the hypothesis that cyclodextrins reversibly enhance nasal absorption of low-molecular-weight heparins (LMWHs) and to investigate the mechanisms by which cyclodextrins enhance LMWH absorption via the nose.
Methods. Absorption of LMWHs was studied by measuring plasma anti-factor Xa activity after nasal administration of various LMWH formulations to anesthetized rats. In vivo reversibility studies were performed to investigate if the effects of cyclodextrins are reversible and diminish with time. The absorption-enhancing mechanisms of cyclodextrins were investigated in cell culture model. The transport of enoxaparin and mannitol, changes in transepithelial electrical resistance (TEER), and distribution of tight junction protein ZO-1 were investigated.
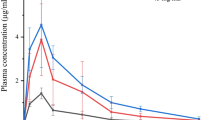
Results. Formulations containing 5% dimethyl-β-cyclodextrin (DMβCD) produced the highest increase in the bioavailability of LMWH preparations tested. In vivo reversibility studies with 5% DMβCD showed that the effect of the absorption enhancer at the site of administration diminished with time. Transport studies using 16HBE14o− cells demonstrated that the increase in the permeability of enoxaparin and mannitol, reduction in TEER, and the changes in the tight junction protein ZO-1 distribution produced by 5% DMβCD were much greater than those produced by β-cyclodextrin (βCD) or hydroxyl-propyl-β-cyclodextrin (HPβCD).
Conclusions. Of the cyclodextrins tested, DMβCD was the most efficacious in enhancing absorption of LMWHs both in vivo and in vitro. The study also suggests that cyclodextrins enhance nasal drug absorption by opening of cell-cell tight junctions.
Similar content being viewed by others
References
Y. W. Chien, K. S. E. Su, and S. Chang. Nasal systemic drug delivery, 1st Ed. Marcel Dekker, New York, 1989.
C. R. Behl, H. K. Pimplaskar, A. P. Sileno, W. J. Xia, W. J. Gries, J. C. deMeireles, and V. D. Romeo. Optimization of systemic nasal drug delivery with pharmaceutical excipients. Adv. Drug Del. Rev 29:117-133 (1998).
M. I. Ugwoke, N. Verbeke, and R. Kinget. The biopharmaceutical aspects of nasal mucoadhesive drug delivery. J. Pharm. Pharmacol. 53:3-22 (2001).
T. Irie, K. Wakamatsu, H. Arima, H. Aritomi, and K. Uekama. Enhancing effects of cyclodextrins on nasal absorption of insulin in rats. Int. J. Pharm. 84:129-139 (1992).
K. Matsubara, K. Abe, T. Irie, and K. Uekama. Im provement of nasal bioavailability of luteinizing hormone-releasing hormone agonist, buserelin, by cyclodextrin derivatives in rats. J. Pharm. Sci. 84:1295-1300 (1995).
Y. Watanabe, Y. Matsumoto, K. Kawamoto, S. Yazawa, and M. Matsumoto. Enhancing effects of cyclodextrins on nasal absorption of insulin and its duration in rabbits. Chem. Pharm. Bull. 40:3100-3104 (1992).
E. Marttin, J. C. Verhoef, and F. W. H. M. Merkus. Efficacy, safety and mechanism of cyclodextrins as absorption enhancers in nasal delivery of peptide and protein drugs. J. Drug Target. 6:17-36 (1998).
R. I. Shulman. Assessment of low-molecular-weight heparin trials in cardiology. Pharm. Ther. 87:1-9 (2000).
J. J. Arnold, F. Ahsan, E. Meezan, and D. J. Pillion. Nasal administration of low molecular weight heparin. J. Pharm. Sci. 91:1707-1714 (2002).
P. Augustijns, P. Annaert, P. Heylen, G. Van den Mooter, and R. Kinget. Drug absorption studies of prodrug ester using the Caco2 model: evaluation of ester hydrolysis and transepithelial transport. Int. J. Pharm. 166:45-53 (1998).
J. Fareed, F. Kaiding, L. H. Yang, and D. A. Hoppensteadt. Pharmacokinetics of low molecular weight heparins in animal models. Semin. Thromb. Hemost. 25:51-55 (1999).
L. Bara and M. Samama. Pharmacokinetics of low molecular weight heparin. Acta Chir. Scand. Suppl. 543:65-72 (1988).
F. Ahsan, J. J. Arnold, T. Yang, E. Meezan, E. M. Schweibert, and D. J. Pillion. gb-cyclodextrin, on insulin movement across human bronchial epithelial cells (16HBE14o-). Eur. J. Pharm. Sci. 20:27-34 (2003).
C. Meaney, B. I. Florea, C. Ehrhardt, U. F. Schäfer, C. Lehr, H. E. Junginger, and G. Borchard. Bronchial epithelial cell cultures. In C. Lehr (ed.), Cell Culture Models of Biological Barriers, In-Vitro Test Systems for Drug Absorption and Delivery, 1st Ed., Taylor and Francis, New York, 2002, pp. 211-227.
T. J. Abbruscato, S. P. Lopez, K. S. Mark, B. T. Hawkins, and T. P. Davis. Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. J. Pharm. Sci. 91:2525-2538 (2002).
F. W. H. M. Merkus, N. G. M. Schipper, W. A. J. J. Hermens, S. G. Romeijn, and J. C. Verhoef. Absorption enhancers in nasal drug delivery: efficacy and safety. J. Controlled Release 24:201-208 (1993).
Physician's Desk Reference. Medical Economic Company Inc., Montvale, NJ, 2002.
E. Marttin, N. G. M. Schipper, J. C. Verhoef, and F. W. H. M. Merkus. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv. Drug Del. Rev. 29:13-38 (1998).
W. A. J. J. Hermens, C. W. J. Belder, J. M. W. M. Merkus, P. M. Hooymans, J. Verhoef, and F. W. H. M. Merkus. Intranasal estradiol administration to oophorectomized women. Eur. J. Obstet. Gynecol. Reprod. Biol. 40:35-41 (1991).
W. A. J. J. Hermens, C. W. J. Belder, J. M. W. M. Merkus, P. M. Hooymans, J. Verhoef, and F. W. H. M. Merkus. Intranasal administration of estradiol in combination with progesterone to oophorectomized women: a pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 43:65-70 (1992).
K. Asai, M. Morishita, H. Datsuta, S. Hosoda, K. Shinomiya, M. Noro, T. Nagai, and K. Takayama. The effects of water-soluble cyclodextrins on the histological integrity of the rat nasal mucosa. Int. J. Pharm. 246:25-35 (2002).
N. Haffejee, J. D. Plessis, D. G. Muller, C. Schultz, A. F. Kotze, and C. Goosen. Intranasal toxicity of selected absorption enhancers. Pharmazie 56:882-888 (2001).
N. Washington, C. Washington, and C. G. Wilson. Physiological Pharmaceutics (Barriers to Drug Absorption), 2nd Ed., Taylor and Francis, New York, 2001, pp. 225.
J. L. Devalia and R. J. Davies. Human nasal and bronchial epithelial cells in culture: an overview of their characteristics and function. Allergy Proc. 12:71-79 (1991).
S. T. Lim, G. P. Martin, M. B. Brown. In vivo and in vitro characterization of novel microparticulates based on hyaluronan and chitosan hydroglutamate. AAPS Pharmsci. Tech. 2(4): article 20 (2001). http://www.pharmscitech.com.
L. Hovagaard and H. Brondsted. Drug delivery studies in Caco-2 monolayer. IV. Absorption enhancer effects of cyclodextrins. Pharm. Res. 12:1328-1332 (1995).
Z. Shao, R. Krishnamoorthy, and A. K. Mitra. Cyclodextrins as nasal absorption promoters of insulin: mechanistic evaluations. Pharm. Res. 9:1157-1163 (1992).
E. Marttin, J. C. Verhoef, S. G. Romeijn, and F. W. H. M. Merkus. Effects of absorption enhancers on rat nasal epithelium in vivo: release of marker compounds in the nasal cavity. Pharm. Res. 12:1151-1157 (1995).
L. Szente. and J. Szejtli. Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv. Drug Del. Rev. 36:17-28 (1999).
K. Uekama and M. Otagiri. Cyclodextrins in drug carrier systems. CRC Crit. Rev. Ther. Drug Carrier Syst. 3:1-40 (1987).
E. Marttin and J. C. Verhoef. C Cullander, S. G. Romeijn, J. F. Nagelkerke and F. W. H. M. Merkus. Confocal laser scanning microscopic visualization of the transport of dextrans after nasal administration to rats: effects of absorption enhancers. Pharm. Res. 14:631-637 (1997).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, T., Hussain, A., Paulson, J. et al. Cyclodextrins in Nasal Delivery of Low-Molecular-Weight Heparins: In Vivo and in Vitro Studies. Pharm Res 21, 1127–1136 (2004). https://doi.org/10.1023/B:PHAM.0000032998.84488.7a
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000032998.84488.7a