Abstract
Purpose. The purpose of this work was to evaluate spray-freeze drying and spray drying processes for encapsulation of darbepoetin alfa (NESP, Aranesp®).
Methods. Darbepoetin alfa was encapsulated in poly(lactide-co-glycolide) by spray-freeze drying and by spray drying. Integrity was evaluated by size-exclusion chromatography and Western blot. Physical properties and in vitro release kinetics were characterized. Pharmacokinetics and pharmacodynamics were evaluated in nude rats.
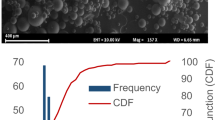
Results. Microspheres produced by spray drying were larger than those produced by spray-freeze drying (69 μm vs. 29 μm). Postencapsulation integrity was excellent for both processes, with <2% dimer by size-exclusion chromatography. In vitro release profiles were similar, with low burst (<25%) and low cumulative protein recovery at 4weeks (≤30%), after which time covalent dimer (≤6.5%) and high molecular weight aggregates (≤2.3%) were recovered by denaturing extraction. After a single injection, darbepoetin alfa was detected in serum through 4 weeks for all microsphere formulations tested in vivo, although relative bioavailability was higher for spray-freeze drying (28%) compared with spray drying (21%; p = 0.08) as were yields (73-82% vs. 34-57%, respectively). For both processes hemoglobin was elevated for 7 weeks, over twice as long as unencapsulated drug.
Conclusions. Spray drying, conducted at pilot scale with commercial equipment, is comparable to spray-freeze drying for encapsulation of darbepoetin alfa.
Similar content being viewed by others
References
S. D. Putney and P. A. Burke. Improving protein therapeutics with sustained-release formulations. Nat. Biotechnol. 16:153-157 (1998).
S. P. Schwendeman, M. Cardomone, M. R. Brandon, A. Klibanov, and R. Langer. Stability of proteins and their delivery from biodegradable polymer microspheres. In S. Cohen and H. Bernstein (eds.), Microparticulate Systems for the Delivery of Proteins and Vaccines, Marcel Dekker, New York, 1996 pp. 1-49.
P. Herbert, K. Murphy, O. Johnson, N. Dong, W. Jaworowicz, M. A. Tracy, J. L. Cleland, and S. D. Putney. A large-scale process to produce microencapsulated proteins. Pharm. Res. 15:357-361 (1998).
P. A. Burke. Controlled release protein therapeutics: Effects of process and formulation on stability. In D. L. Wise (ed.), Handbook of Pharmaceutical Controlled Release Technology, Marcel Dekker, New York, 2000, pp. 661-692.
J. L. Cleland, O. L. Johnson, S. Putney, and A. J. S. Jones. Recombinant human growth hormone poly(lactic-co-glycolic acid) microsphere formulation development. Adv. Drug Deliv. Rev. 28:71-84 (1997).
P. Giunchedi and U. Conte. Spray-drying as a preparation method of microparticulate drug delivery systems: an overview. S T P Pharma Sci. 5:276-290 (1995).
F. X. Lacasse, P. Hildgen, J. Perodin, E. Escher, N. C. Phillips, and J. N. McMullen. Improved activity of a new angiotensin receptor antagonist by an injectable spray-dried polymer microsphere preparation. Pharm. Res. 14:887-891 (1997).
T. Kissel, Z. Brich, S. Bantle, I. Lancranjan, F. Nimmerfall, and P. Vit. Parenteral depot-systems on the basis of biodegradable polyesters. J. Control. Release 16:27-42 (1991).
K. Masters. Spray Drying in Practice, SprayDryConsult International ApS, Charlottenlund, Denmark, 2002.
P. Giunchedi, B. Conti, I. Genta, U. Conte, and G. Puglisi. Emulsion spray-drying for the preparation of albumin-loaded PLGA microspheres. Drug Devel. Ind. Pharm. 27:745-750 (2001).
B. Bittner and T. Kissel. Ultrasonic atomization for spray drying: a versatile technique for the preparation of protein loaded biodegradable microspheres. J. Microencap. 16:325-341 (1999).
B. Baras, M. A. Benoit, and J. Gillard. Parameters influencing the antigen release from spray-dried poly (DL-lactide) microparticles. Int. J. Pharm. 200:133-145 (2000).
B. Baras, M. A. Benoit, G.-O. Poulain, A. M. Schacht, A. Capron, J. Gillard, and G. Riveau. Vaccine properties of antigens entrapped in microparticles produced by spray-drying technique and using various polyester polymers. Vaccine 18:1495-1505 (2000).
B. Bittner, M. Morlock, H. Koll, G. Winter, and T. Kissel. Recombinant human erythropoietin (rhEPO) loaded poly(lactide-co-glycolide) microspheres: influence of the encapsulation technique and polymer purity on microsphere characteristics. Eur. J. Pharm. Biopharm. 45:295-305 (1998).
C. Perez, I. J. Castellanos, H. R. Costantino, W. Al-Azzam, and K. Griebenow. Recent trends in stabilizing protein structure upon encapsulation and release from bioerodible polymers. J. Pharm. Pharmacol. 54:301-313 (2002).
H. T. Wang, H. Palmer, R. J. Linhardt, D. R. Flanagan, and E. Schmitt. Degradation of poly(ester) microspheres. Biomaterials 11:679-685 (1990).
E. Mathiowitz, H. Bernstein, S. Giannos, P. Dor, T. Turek, and R. Langer. Polyanhydride microspheres. IV. Morphology and characterization of systems made by spray drying. J. Appl. Polym. Sci. 45:125-134 (1992).
S. B. Krantz. Erythropoietin. Blood 77:419-434 (1991).
J. C. Egrie and J. K. Browne. Development and characterization of novel erythropoiesis stimulating protein (NESP). Br. J. Cancer 84(Supp 1):3-10 (2001).
X. C. Nguyen, J. D. Herberger, and P. A. Burke. Protein powders for encapsulation: a comparison of spray-freeze drying and spray drying of darbepoetin alfa. Pharm. Res. 21:507-514 (2004).
O. L. Johnson, W. Jaworowicz, J. L. Cleland, L. Bailey, M. Charnis, E. Duenas, C. C. Wu, D. Shepard, S. Magil, T. Last, A. J. S. Jones, and S. D. Putney. The stabilization and encapsulation of human growth-hormone into biodegradable microspheres. Pharm. Res. 14:730-735 (1997).
J. Herberger, K. Murphy, L. Munyakazi, J. Cordia, and E. Westhaus. Carbon dioxide extraction of residual solvents in poly(lactide-co-glycolide) microparticles. J. Control. Rel. 90:181-195 (2003).
A. G. Hausberger and P. P. DeLuca. Characterization of biodegradable poly(D,L-lactide-co-glycolide) polymers and microspheres. J. Pharm. Biomed. Anal. 13:747-760 (1995).
U. S. Pharmacopeaia and National Formulary, United States Pharmacopeial Convention, Inc., Rockville, MD, 1999.
X. M. Lam, E. T. Duenas, A. L. Daugherty, N. Levin, and J. L. Cleland. Sustained release of recombinant human insulin-like growth factor-I for treatment of diabetes. J. Control. Rel. 67:281-292 (2000).
Y. Ogawa. Injectable microcapsules prepared with biodegradable poly(alpha-hydroxy) acids for prolonged release of drugs. J. Biomater. Sci. Polym. Ed. 8:391-409 (1997).
N. Clarke, K. O'Connor, and Z. Ramtoola. Influence of formulation variables on the morphology of biodegradable microparticles prepared by spray drying. Drug Devel. Ind. Pharm. 24:169-174 (1998).
M. Morlock, H. Koll, G. Winter, and T. Kissel. Microencapsulation of rh-erythropoietin, using biodegradable poly(d,l-lactide-co-glycolide): protein stability and the effects of stabilizing excipients. Eur. J. Pharm. Biopharm. 43:29-36 (1997).
M. A. Tracy, K. L. Ward, L. Firouzabadian, Y. Wang, N. Dong, R. Qian, and Y. Zhang. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials 20:1057-1062 (1999).
T. Heya, H. Okada, Y. Ogawa, and H. Toguchi. In vitro and in vivo evaluation of thyrotropin releasing hormone release from co-poly(dl-lactic/glycolic acid) microspheres. J. Pharm. Sci. 83:636-640 (1994).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burke, P.A., Klumb, L.A., Herberger, J.D. et al. Poly(Lactide-Co-Glycolide) Microsphere Formulations of Darbepoetin Alfa: Spray Drying Is an Alternative to Encapsulation by Spray-Freeze Drying. Pharm Res 21, 500–506 (2004). https://doi.org/10.1023/B:PHAM.0000019305.79599.a5
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000019305.79599.a5