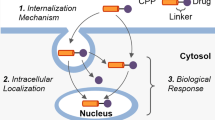
Abstract
Protein transduction domains (PTDs) are small cationic peptides that can facilitate the uptake of large, biologically active molecules into mammalian cells. Recent reports have suggested that PTDs may be able to mediate the delivery of cargo to tissues throughout a living organism. Such technology could eliminate the size restrictions on usable drugs, enabling previously unavailable large molecules to modulate in vivo biology and alleviate disease. In this article, we review the evidence that PTDs can be used both to deliver active molecules to pathological tissue in vivo and to treat models of disease such as ischemia, inflammation, and cancer.
Similar content being viewed by others
References
M. A. Lindsay. Peptide-mediated cell delivery: application in protein target validation. Curr. Opin. Pharmacol. 2:587-594 (2002).
P. Fischer, E. Krausz, and D. P. Lane. Cellular delivery of impermeable effector molecules in the form of conjugates with peptides capable of mediating membrane translocation. Bioconjug. Chem. 12:825-841 (2001).
S. R. Schwarze, A. Ho, A. Vocero-Akbani, and S. F. Dowdy. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285:1569-1572 (1999).
A. D. Frankel and C. O. Pabo. Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55:1189-1193 (1988).
M. Green and P. M. Loewenstein. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 55:1179-1188 (1988).
E. Vives, P. Brodin, and B. Lebleu. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 272:16010-16017 (1997).
D. Derossi, A. H. Joliot, G. Chassaing, and A. Prochiantz. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 269:10444-10450 (1994).
J. C. Mai, H. Shen, S. C. Watkins, T. Cheng, and P. D. Robbins. Efficiency of protein transduction is cell type-dependent and is enhanced by dextran sulfate. J. Biol. Chem. 277:30208-30218 (2002).
P. A. Wender, D. J. Mitchell, K. Pattabiraman, E. T. Pelkey, L. Steinman, and J. B. Rothbard. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters. Proc. Natl. Acad. Sci. U. S. A. 97:13003-13008 (2000).
H. Nagahara, A. M. Vocero-Akbani, E. L. Snyder, A. Ho, D. G. Latham, N.A. Lissy, M. Becker-Hapak, S. A. Ezhevsky, and S. F. Dowdy. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat. Med. 4:1449-1452 (1998).
M. Lewin, N. Carlesso, C. H. Tung, X. W. Tang, D. Cory, D. T. Scadden, and R. Weissleder. Tat peptide-derivatized magnetic nanoparticles allow in vivo tracking and recovery of progenitor cells. Nat. Biotechnol. 18:410-414 (2000).
A. Eguchi, T. Akuta, H. Okuyama, T. Senda, H. Yokoi, H. Inokuchi, S. Fujita, T. Hayakawa, K. Takeda, M. Hasegawa, and M. Nakanishi. Protein transduction domain of HIV-1 Tat protein promotes efficient delivery of DNA into mammalian cells. J. Biol. Chem. 276:26204-26210 (2001).
V. P. Torchilin, R. Rammohan, V. Weissig, and T. S. Levchenko. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc. Natl. Acad. Sci. U. S. A. 98:8786-8791 (2001).
V. P. Torchilin, T. S. Levchenko, R. Rammohan, N. Volodina, B. Papahadjopoulos-Sternberg, and G. G. D'Souza. Cell transfection in vitro and in vivo with nontoxic TAT peptide-liposome-DNA complexes. Proc. Natl. Acad. Sci. U. S. A. 100:1972-1977 (2003).
D. Derossi, S. Calvet, A. Trembleau, A. Brunissen, G. Chassaing, and A. Prochiantz. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J. Biol. Chem. 271:18188-18193 (1996).
M. Tyagi, M. Rusnati, M. Presta, and M. Giacca. Internalization of HIV-1 Tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 276:3254-3261 (2001).
M. Silhol, M. Tyagi, M. Giacca, B. Lebleu, and E. Vives. Different mechanisms for cellular internalization of the HIV-1 Tat-derived cell penetrating peptide and recombinant proteins fused to Tat. Eur. J. Biochem. 269:494-501 (2002).
J. P. Richard, K. Melikov, E. Vives, C. Ramos, B. Verbeure, M. J. Gait, L. V. Chernomordik, and B. Lebleu. Cell-penetrating peptides: a re-evaluation of the mechanism of cellular uptake. J. Biol. Chem. 278:585-590 (2002).
A. Fittipaldi, A. Ferrari, M. Zoppe, C. Arcangeli, V. Pellegrini, F. Beltram, and M. Giacca. Cell membrane lipid rafts mediate caveolar endocytosis of HIV-1 TAT fusion proteins. J. Biol. Chem 278:34141-34149 (2003).
S. Violini, V. Sharma, J. L. Prior, M. Dyszlewski, and D. Piwnica-Worms. Evidence for a plasma membrane-mediated permeability barrier to Tat basic domain in well-differentiated epithelial cells: lack of correlation with heparan sulfate. Biochemistry 41:12652-12661 (2002).
G. Cao, W. Pei, H. Ge, Q. Liang, Y. Luo, F. R. Sharp, A. Lu, R. Ran, S. H. Graham, and J. Chen. In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J. Neurosci. 22:5423-5431 (2002).
E. Kilic, G. P. H. Dietz, D. M. Hermann, and M. Bahr. Intravenous TAT-Bcl-xL is protective after middle cerbral artery occlusion in mice. Ann. Neurol. 52:617-622 (2002).
G. P. H. Dietz, E. Kilic, and M. Bahr. Inhibition of neuronal apoptosis in vitro and in vivo using TAT-mediated protein transduction. Mol. Cell. Neurosci. 21:29-37 (2002).
S. Asoh, I. Ohsawa, T. Mori, K. Katsura, T. Hiraide, Y. Katayama, M. Kimura, D. Ozaki, K. Yamagata, and S. Ohta. Protection against ischemic brain injury by protein therapeutics. Proc. Natl. Acad. Sci. U. S. A. 99:17107-17112 (2002).
J. Embury, D. Klein, A. Pileggi, M. Ribeiro, S. Jayaraman, R. D. Molano, C. Fraker, N. Kenyon, C. Ricordi, L. Inverardi, and R. L. Pastori. Proteins linked to a protein transduction domain efficiently transduce pancreatic islets. Diabetes 50:1706-1713 (2001).
M. Aarts, Y. Liu, L. Liu, S. Besshoh, M. Arundine, J. W. Gurd, Y. T. Wang, M. W. Salter, and M. Tymianski. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 298:846-850 (2002).
L. Chen, H. Hahn, G. Wu, C. H. Chen, T. Liron, D. Schechtman, G. Cavallaro, L. Banci, Y. Guo, R. Bolli, G. W. Dorn 2nd, and D. Mochly-Rosen. Opposing cardioprotective actions and parallel hypertrophic effects of deltaPKC and epsilonPKC. Proc. Natl. Acad. Sci. U. S. A. 98:11114-11119 (2001).
A. B. Gustafsson, M. R. Sayen, S. D. Williams, M. T. Crow, and R. A. Gottlieb. TAT protein transduction into isolated perfused hearts. Circulation 106:735-739 (2002).
C. R. Aarnt, M. V. Chiorean, M. P. Heldebrant, G. J. Gores, and S. Kaufman. Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J. Biol. Chem. 277:44236-44243 (2002).
S. Fulda, W. Wick, M. Weller, and K-M. Debatin. Smac agonists sensitize for Apo2L/TRAIL-or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat. Med. 8:808-815 (2002).
D. Vucic, K. Deshayes, H. Ackerly, M. T. Pisabarro, S. Kadkhodayan, W. J. Fairbrother, and W. M. Dixit. SMAC negatively regulates the anti-apoptotic activity of melanoma inhibitor of apoptosis (ML-IAP). J. Biol. Chem. 277:12275-12279 (2002).
J. W. Harbour, L. Worley, D. Ma, and M. Cohen. Transducible peptide therapy for uveal melanoma and retinoblastoma. Arch. Ophthalmol. 120:1341-1346 (2002).
J. C. Mai, Z. Mi, S-H. Kim, B. Ng, and P. D. Robbins. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 61:7709-7712 (2001).
H. Harada, M. Hiraoka, and S. Kizaka-Kondoh. Antitumor effect of TAT-Oxygen-dependent Degradation-Caspase-3 fusion protein specifcally stabilized and activated in hypoxic tumor cells. Cancer Res. 62:2013-2018 (2002).
K. Datta, C. Sundberg, S. A. Karumanchi, and D. Mukhopadhyay. The 104-123 amino acid sequence of the beta-domain of von Hippel-Lindau gene product is sufficient to inhibit renal tumor growth and invasion. Cancer Res. 61:1768-1775 (2001).
R. Hosotani, Y. Miyamoto, K. Fujimoto, R. Doi, A. Otaka, N. Fujii, and M. Imamura. Trojan p16 peptide suppresses pancreatic cancer growth and prolongs survival in mice. Clin. Cancer Res. 8:1271-1276 (2002).
F. E. Rey, M. E. Cifuentes, A. Kiarash, M. T. Quinn, and P. J. Pagano. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O2-and systolic blood pressure in mice. Circ. Res. 89:408-414 (2001).
M. J. May, F. D'Acquisto, L. A. Madge, J. Glockner, J. S. Pober, and S. Ghosh. Selective inhibition of NF-kB activation by a peptide that blocks the interaction of NEMO with the IkB Kinase complex. Science 289:1550-1554 (2000).
M. Bucci, J. P. Gratton, R. D. Rudic, L. Acevedo, F. Roviezzo, G. Cirino, and W. C. Sessa. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat. Med. 6:1362-1367 (2000).
J. B. Rothbard, S. Garlington, Q. Lin, T. Kirschberg, E. Kreider, P. L. McGrane, P. A. Wender, and P. A. Khavari. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nat. Med. 6:1253-1257 (2000).
C. Rousselle, P. Clair, J. M. Lefauconnier, M. Kaczorek, J. M. Scherrmann, and J. Temsamani. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide-vector-mediated strategy. Mol. Pharmacol. 57:679-686 (2000).
M. Mazel, P. Clair, C. Rousselle, P. Vidal, J. M. Scherrmann, D. Mathieu, and J. Temsamani. Doxorubicin-peptide conjugates overcome multidrug resistance. Anticancer Drugs 12:107-116 (2001).
H. J. Lee and W. M. Pardridge. Pharmacokinetics and delivery of Tat and Tat-protein conjugates to tissues in vivo. Bioconjug. Chem. 12:995-999 (2001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Snyder, E.L., Dowdy, S.F. Cell Penetrating Peptides in Drug Delivery. Pharm Res 21, 389–393 (2004). https://doi.org/10.1023/B:PHAM.0000019289.61978.f5
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000019289.61978.f5