Abstract
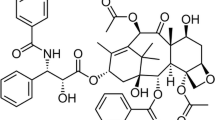
Purpose. We successfully manufactured nanoparticles of biodegradable polymers for controlled release of paclitaxel. TPGS (d-α-tocopheryl polyethylene glycol 1000 succinate) could be a novel material to make nanoparticles of high drug encapsulation efficiency (EE) and desired physicochemical and pharmaceutical properties of the drug loaded nanoparticles. Among various controlling parameters in the process, the present work is to elucidate the effects of the surfactant stabilizer and the drug loading ratio.
Methods. Paclitaxel loaded PLGA nanoparticles were formulated at various drug-loading ratios by a modified single emulsion solvent extraction/evaporation technique. TPGS was introduced either as the emulsifier or as a matrix material component by using different technique. Polyvinyl alcohol (PVA) was also used for a comparison. The nanoparticles of various recipes were characterized by various state-of-the-art instrument technology for their properties.
Results. The EE and the in vitro release behavior were found significantly influenced by the drug loading ratio and the surfactant stabilizer encountered. TPGS involved nanoparticles can have high EE and other favorable properties.
Conclusions. TPGS could be a novel and effective emulsifier, which can result in high EE and desired properties of paclitaxel-loaded polymeric nanoparticles.
Similar content being viewed by others
References
K. S. Soppimath, T. M. Aminabhavi, A. R. Kulkarni and W. E. Rudzinski, Biodegradable polymeric nanoparticles as drug delivery devices, J. Control Rel. 70:1-20 (2001).
S. M. Moghimi, A. C. Hunter, and J. C. Murray. Long-circulating and target specific nanoparticles: theory to practice. Pharmacol. Rev. 53:283-318 (2001).
R. Langer. Biomaterials in drug delivery and tissue engineering: one laboratory's experience. Acc. Chem. Res. 33 (2):94-101 (2000).
C. X. Songa, V. Labhasetwara, H. Murphya, X. Qua, W. R. Humphreyb, R. J. Shebuskib and R. J. Levy, Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery, J. Control Rel. 43:197-212 (1997).
V. Labhasetwar. Nanoparticles for drug delivery. Pharm. News. 4:28-31 (1997).
J. Couzin. Cancer Research: nanoparticles cut tumors. Science 296:2314-2315 (2002).
J. D. Hood, M. Bednarski, R. Frausto, S. Guccione, R. A. Reisfeld, R. Xiang, and D. A. Cheresh. Tumor Regression by Targeted Gene Delivery to the Neovasculature. Science 296:2404-2407 (2002).
V. Labhasetwar, C. Song, and R. J. Levy. Nanoparticle drug delivery system for restenosis. Adv. Drug Delivery Rev. 24:63-85 (1997).
R. H. Muller. (ed.), Colloidal Carriers for Controlled Drug Delivery and Targeting, CRC Press, Boca Raton, Florida. 1991, pp. 1-16.
P. D. Scholes, A. G. A. Coombes, L. Illum, et al. The preparation of sub-200 nm poly (lactide-co-glycolide) microspheres for site-specific drug delivery. J. Control Rel. 25:145-153 (1993).
M.F. Zambaux, F. Bonneaux, R. Gref et al. Influence of experimental parameters on the characteristics of poly (lactic acid) nanoparticles prepared by a double emulsion method. J. Control. Rel. 50:31-40 (1998).
A. Carrio, G. Schwach, J. Coudane, and M. Vert. Preparation and degradation of surfactant-free PLGA microspheres. J. Control Rel. 37:113-121 (1991).
S. C. Lee, J. T. Oh, M. H. Jang, and S. Chung. Quantitative analysis of polyvinyl alcohol on the surface of poly(D,L-lactide-co-glycolide) microparticles prepared by solvent evaporation method: effect of particle size and PVA concentration. J. Control Rel. 59:123-132 (1999).
D. T. Birnbaum, J. D. Kosmala, and L. Brannon-Peppas. Optimization of preparation techniques for poly(lactic acid-co-glycolide acid) nanoparticles. J. Nanoparticle Res. 2:173-181 (2000).
D. Quintanar-Guerrero, H. Fessi, E. Allemann, and E. Doelker. Influence of stabilizing agents and preparative variables on the formation of poly(D,L-lactic acid) nanoparticles by an emulsification-diffusion technique. Int. J. Pharmaceutics 143:133-141 (1996).
M. F. Zambaux, F. Bonneaux, R. Gref, P. Maincent, E. Dellacherie, M. J. Alonso, P. Labrude, and C. Vigneron. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. J. Control Rel. 50:31-40 (1998).
R. Gref, V. Babak, P. Bouillot, I. Lukina, M. Bodorev, and E. Dellacherie. Interfacial and emulsion stabilizing properties of amphiphilic water-soluble poly(ethylene glycol)-poly(lactic acid) copolymers for the fabrication of biocompatible nanoparticles. Colloids and Surfaces. A Physicochemical and Engineering Aspects. 143:413-420 (1998).
S. K. Sahoo, J. Panyam, S. Prabha, and V. Labhasetwar. Residual polyvinyl alcohol associated with poly (D,L-lactide-co-glycolide) nanoparticles affects their physical properties and cellular uptake. J. Controlled Release 82 (1):105-114 (2002).
A. K. Singla, G. Alka, and A. Deepika. Paclitaxel and its formulations. Int. J. Pharmaceutics 235:179-192 (2002).
E. Tatou, C. Mossiat, V. Maupoil, F. Gabrielle, M. David, and L. Rochette. Effects of cyclosporin and cremophor on working rat heart and incidence of myocardial lipid peroxidation. Pharmacol. 52:1-7 (1996).
R. T. Dorr. Pharmacology and toxicology of Cremophor EL diluent. Ann. Pharmacother. 28:S11-S14 (1994).
M. L. Fjallskog, L. Frii, and J. Bergh. Is cremophor, solvent for paclitaxel, cytotoxic? Lancet 342:873(1993).
L. Mu and S. S. Feng. A novel controlled release formulation for anticancer drug paclitaxel (Taxol\R): PLGA nanoparticles containing vitamin E TPGS. J. Control. Rel. 86:33-48 (2003).
Y. N. Konan, R. Gurny, and E. Allemann. Preparation and characterization of sterile and freeze-dried sub-200 nm nanoparticles. Int. J. Pharmaceutics 233:239-252 (2002).
H. Murakami, Y. Kawashima, T. Niwa, T. Hino, H. Takeuchi, and M. Kobayashi. Influence of the degrees of hydrolyzation and polymerization of poly(vinylalcohol) on the preparation and properties of poly(DL-lactide-co-glycolide) nanoparticle. Int. J. Pharmaceutics 149:43-49 (1997).
H. Murakami, M. Kobayashi, H. Takeuchi, and Y. Kawashima. Preparation of poly(DL-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int. J. Pharmaceutics 187:143-152 (1999).
C. Dubernet. Thermoanalysis of microspheres. Thermochimica Acta 248:259-269 (1995).
P. D. Scholes, A. G. A. Coombes, L. Illum, S. S. Davis, J. F. Watts, C. Ustariz, M. Vert, and M. C. Davies. Detection and determination of surface levels of poloxamer and PVA surfactant on biodegradable nanospheres using SSIMS and XPS. J. Control Rel. 59:261-278 (1999).
D. Briggs and M. P. Seah. (eds), Practical Surface Analysis by Auger and X-ray Photoelectron Spectroscopy, John Wiley, Chi-chester, 1990.
T. Gorner, R. Gref, D. Michenot, F. Sommer, M. N. Tran, and E. Dellacherie. Lidocaine-loaded biodegradable nanospheres. I. Optimization of the drug incorporation into the polymer matrix. J. Control. Rel. 57:259-268 (1999).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mu, L., Feng, SS. PLGA/TPGS Nanoparticles for Controlled Release of Paclitaxel: Effects of the Emulsifier and Drug Loading Ratio. Pharm Res 20, 1864–1872 (2003). https://doi.org/10.1023/B:PHAM.0000003387.15428.42
Issue Date:
DOI: https://doi.org/10.1023/B:PHAM.0000003387.15428.42