Abstract
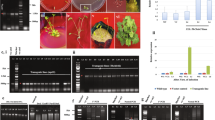
Lignin is a complex aromatic polymer of vascular plants that provides mechanical strength to the stem and protects cellulose fibres from chemical and biological degradation. 4-Coumarate : CoA ligases (EC 6.2.1.12) are key enzymes for the biosynthetic pathway of monolignols which is an important complex aromatic polymer for lignin biosynthesis and tree growth. Recently, 4-coumarate : CoA ligase has been used as exogenous gene in transgenic plants to genetically modify the lignin biosynthesis pathway. Since most lignin is produced in the vascular cells, a tissue-specific-expressed promoter in the vascular cell would be important and useful to change and modify the content of lignin. Here we report the existence of a promoter of GRP1.8 (the glycine-rich protein 1.8) in Sopho japonica L. (GenBank accession number AF250149) and studies on its function in transgenic tobacco. The promoter activity was analyzed in transgenic tobacco plants by histochemical staining of GUS gene expression driven by a 613-bp sjGRP1.8p promoter sequence. In sjGRP1.8p-GUS transgenic plants, intense GUS staining was detected in the xylem of the stem. To further investigate the regulation of the tissue-specific expression of the 4CL1 gene, we analyzed the activity of the 4CL1 gene which is sense orientated with the sjGRP1.8p promoter in transgenic tobacco. The Pto4CL1 gene was expressed in the stem of transgenic tobacco. The activity of the 4CL1 enzyme was increased 1–2-fold in the stem but not increased in the leaves of transgenic tobacco. In comparison with the control plants, the content of lignin was increased 25% in the stem but there was no increase in the leaves of transgenic tobacco.
Similar content being viewed by others
References
Allina S.M., Aviva P.H., Theilman D.A., Ellis B.E. and Douglas C.J. 1998. 4-coumarate:coenzyme A ligase in hybrid popar. Plant Physiol. 116: 743–754.
Atanassova R., Favet N., Martz F., Chabbert B., Tollier M.T., Monties B., Fritig B. and Legrand M. 1995. Altered lignin composition in transgenic tobacco expressing o-methyltransferase sequences in sense and antisense orientation. Plant J. 8: 465–477.
Baucher M., Chabbert B., Pilate G., Van Doorsselaere J., Tollier M.T., Petitconil M., Cornu D., Monties B., Van motagu M. and Inze D. 1996. Red xylem and higher lignin extractability by down-regulating a cinnamyl alcohol dehydrogenase in poplar. Plant Physiol. 112: 1479–1490.
Bell-Lelong D.A., Cousumano J.C., Meyer K. and Chapple C. 1997. Cinnamate-4-hydroxylase expression in Arabidopsisregulation in response to development and the environment. Plant Physiol. 113: 729–738.
Bevan M., Shufflebottom D., Edwards K., Jefferson R. and Schuch W. 1989. Tissue-and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. EMBO J. 8: 1899–1906.
Bogos R.C., Chiang V.L., Zhang X.H., Campbell E.R., Podila G.K. and Campbell W.H. 1995. RNA isolation from plant tissues recalcitrant to extraction in guandine. Biotechniques 19: 734–737.
Broadford M.M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248–254.
Church G.M. and Gilbert W. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. USA 81: 1991–1995.
Dwivedi U.N., Campbell W.H., Yu J., Datla R.S., Bugos R.C., Chiang V.L. and Podila G.K. 1994. Modification of lignin biosynthesis in transgenic Nicotiana through expression of an antisense O-methyltransferase gene from Populus. Plant Mol. Biol. 26: 61–71.
Halpin C., Mary E.K., Geoffery A.F., Malcolm M.C., Alain M.B., Jaap J.B., Brigitte C., Marie-Therese T. and wolfgang S. 1994. Manipulation of lignin quality by downregulation of cinnamyl alcohol dehydrogenase. Plant J. 6: 339–350.
Hibino T., Takabe K., Kawazu T., Shibata D. and Higuchi T. 1995. Increase of cinnamaldehyde groups in lignin of transgenic tobacco plants carrying an antisense gene for cinnamyl alcohol dehydrogenase. Biosci. Biotech. Biochem. 59: 929–931.
Higuchi T. 1997. In: Biochemistry and Molecular Biology of Wood. Springer, New York, pp. 131–233.
Holsters M., De W.D., Depicker A., Messens E., Van M.M. and Schell J. 1978. Transfection and transformation of Agrobacterium tumefaciens. Mol. Gen. Genet. 163: 181–187.
Jefferson R.A. 1987. Assaying chimeric genes in transgenic plants: the GUS gene fusion system. Plant Mol. Biol. Rep. 5: 387–405.
Keller B., Templeton M.D. and Lamb C.J. 1989. Specific localization of a plant cell wall glycine-rich protein in protoxylem cells of the xylem system. Proc. Natl. Acad. Sci. USA 86: 1529–1533.
Keller B., Nierhaus-Wunderwald D. and Amrhein N. 1990. Deposition of glycine-rich structural protein in xylem cell walls of french bean seedling is independent of lignification. J. Struct. Biol. 104: 144–149.
Keller B. and Christine B. 1991. Vascular-specific expression of the bean GRP 1.8 gene is negatively regulated. Plant Cell 3: 1051–1061.
Keller B. and Heierli D. 1994. Vascular expression of the grp1.8 promoter is controlled by three specific regulatory elements and one unspecific activating sequence. Plant Mol. Biol. 26(2): 747–756.
Knobloch K.H. and Hahlbrock K. 1975. Isoenzymes of p-coumarate: CoA ligase from cell suspension cultures of Glycine max. Eur. J. Biochem. 52: 311–320.
Knobloch K.H. and Hanlbrock K. 1977. 4-Coumarate:CoA ligase form cell suspension cultures of Petroselinum hortense. Hoffm. Arch. Biochem. Biophys. 184: 237–248.
Lee D., Ellard M.E., Wanner L.A., Davis K.R. and Douglas C.J. 1995. The arabidopsis 4-coumarate:CoA ligase(4CL) gene and developmentally regulated expression and nucleotide sequence of its cDNA. Plant Mol. Biol. 28: 871–884.
Li L., Popko J.L., Zhang X.-H., Osakabe K., Tsai C.J., Joshi C.P. and Chiang V.L. 1997. A novel multifunctional O-methyltransferase implicated in a dual methylation pathway associated with lignin biosynthesis in loblolly pine. Proc. Natl. Acad. Sci. USA 94: 5461–5466.
Ringli C., Keller B. and Ryser U. 2001a. Glycine-rich proteins as structural components of plant cell walls. Cell Mol. Life Sci. 58(10): 1430–1441.
Ringli C., Hauf G. and Keller B. 2001b. Hydrophobic interactions of the structural protein grp1.8 in the cell wall of protoxylem elements. Plant Physiol. 125(2): 673–682.
Sakuta C. and Satoh S. 2000. Vascular tissue-specific gene expression of xylem sap glycine-rich proteins in root and their localization in the walls of metaxylem vessels in cucumber. Plant Cell Physiol. 41(5): 627–638.
Sambrook J., Fritsch E.F. and Maniatis T. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
Torres-Schumann S., Ringli C., Heierli D., Amrhein N. and Keller B. 1996. In vitro binding of the tomato bZIP transcriptional activator VSF-1 to a regulatory element that controls xylem-specific gene expression. Plant J. 9(3): 283–296.
Tsai C.J., Podila G.K. and Chiang V.L. 1994. Agrobacteriummediated transformation of quaking aspen (populus tremulodies) and regeneration of transgenic plants. Plant Cell Rep. 14: 94–97.
Tsai C.J., Popko J.L., Moelke M.R., Hu W.J., Podila G.K. and Chiang V.L. 1998. Suppression of O-methyltransferase gene by homologous sense transgene in quaking aspen causes redbrown wood phenotypes. Plant Physiol. 117: 101–112.
Van Doorsselaere D.J. 1995. Novel lignin in poplar trees with a reduced caffeic acid/5-hydroxyferulic acid O-methyltransferase activity. Plant J. 8: 855–864.
Wang G.L. and Fang H.J. 1998. Plant Genetic Engineering Theory and Technology, Science Press, Beijing, pp. 600–601.
Wen-jing H., Scott A.H., Jahau L., Jacqueeline L.P., John R., Stokke D.D., Tsai C.J. and Vincent L.C. 1999. Repression of lignin biosynthesis promotes cellulose accumulation and growth in transgenic trees. Nat. Biotechnol. 17: 808–812.
Whetten R.W., Mackay J.J. and Sederoff R.R. 1998. Present advances in understanding lignin biosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49: 585–609.
Ye Z.H. and Varner J.E. 1991. Tissue-specific expression of cell wall proteins in developing soybean tissues. Plant Cell 3: 23–27.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, H., Zeng, Q., Zhao, Y. et al. Xylem-specific expression of a GRP1.8 promoter::4CL gene construct in transgenic tobacco. Plant Growth Regulation 41, 279–286 (2003). https://doi.org/10.1023/B:GROW.0000007507.98276.1d
Issue Date:
DOI: https://doi.org/10.1023/B:GROW.0000007507.98276.1d