Abstract
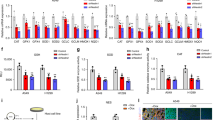
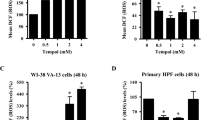
Transient/chronic microenvironmental hypoxia that exists within a majority of solid tumors has been suggested to have a profound influence on tumor growth and therapeutic outcome. Since the functions of novel antioxidant proteins, peroxiredoxin I (Prx I) and II, have been implicated in regulating cell proliferation, differentiation, and apoptosis, it was of our special interest to probe a possible role of Prx I and II in the context of hypoxic tumor microenvironment. Since both Prx I and II use thioredoxin (Trx) as an electron donor and Trx is a substrate for thioredoxin reductase (TrxR), we investigated the regulation of Trx and TrxR as well as Prx expression following hypoxia. Here we show a dynamic change of glutathione homeostasis in lung cancer A549 cells and an up-regulation of Prx I and Trx following hypoxia. Western blot analysis of 10 human lung cancer and paired normal lung tissues also revealed an elevated expression of Prx I and Trx proteins in lung cancer tissues. Immunohistochemical analysis of the lung cancer tissues confirmed an augmented Prx I and Trx expression in cancer cells with respect to the parenchymal cells in adjacent normal lung tissue. Based on these results, we suggest that the redox changes in lung tumor microenvironment could have acted as a trigger for the up-regulation of Prx I and Trx in lung cancer cells. Although the clinical significance of our finding awaits more rigorous future study, preferential augmentation of the Prx I and Trx in lung cancer cells may well represent an attempt of cancer cells to manipulate a dynamic redox change in tumor microenvironment in a manner that is beneficial for their proliferation and malignant progression.
Similar content being viewed by others
References
Azzam EI, De Toledo SM, Spitz DR, Little JB. Oxidative metabolism modulates signal transduction and micronucleus formation in bystander cells from alpha-particle-irradiated normal human fibroblast cultures. Cancer Res. 2002;62(19):5436–42.
Bae YS, Kang SW, Seo MS, et al. Epidermal growth factor (EGF)-induced generation of hydrogen peroxide. J Biol Chem. 1997;272:217–21.
Baek SH, Han MY, Lee SH, Choi EM, Park YM. Hypoxic micro-environmental control of stress protein and erythropoietin gene expression. J Biochem Mol Biol. 1999;32:112–18.
Baek SH, Lee UY, Park EM, Han MY, Lee YS, Park YM. Role of protein kinase C delta in transmitting hypoxia signal to HSF and HIF-1. J Cell Physiol. 2001;188:223–35.
Baek SH, Min JN, Park EM, et al. Role of small heat shock protein HSP25 in radioresistance and glutathione-redox cycle. J Cell. Physiol. 2000;183:100–7.
Berggren MI, Husbeck B, Samulitis B, Baker AF, Gallegos A, Powis G. Thioredoxin peroxidase-1 (peroxiredoxin-1) is increased in thioredoxin-1 transfected cells and results in enhanced protection against apoptosis caused by hydrogen peroxide but not by other agents including dexamethasone, etoposide, and doxorubicin. Arch Biochem Biophys. 2001;392:103–9.
Bishopric NH, Andreka P, Slepak T, Webster KA. Molecular mechanisms of apoptosis in the cardiac myocyte. Curr Opin Pharmacol. 2001;1:141–50.
Brizel DM, Scully SP, Harrelson JM, et al. Tumor oxygenation predicts for the likelihood of distant metastasis in human soft tissue sarcoma. Cancer Res. 1996;56:941–3.
Brizel DM, Sibley GS, Prosnitz, LR, Scher RL, Dewhirst MW. Tumor hypoxia adversely affects the prognosis of carcinoma of the head and neck. Int J Radiat Oncol Biol Phys. 1997;38:285–9.
Brown JM. Evidence for acutely hypoxic cells in mouse tumours and a possible mechanism of reoxygenation. Br J Radiol. 1979;52:650–6.
Brown NS, Bicknell R. Hypoxia and oxidative stress in breast cancer. Oxidative stress: its effects on the growth, metastatic potential and response to therapy of breast cancer. Breast Cancer Res. 2001;3:323–7.
Butterfield LH, Merino A, Golub SH, Shau H. From cytoprotection to tumor suppression: the multifactorial role of peroxiredoxins. Antioxid Redox Signal. 1999;1:385–402.
Chae HZ, Kim HJ, Kang SW, Rhee SG. Characterization of three isoforms of mammalian peroxiredoxin that reduce peroxides in the presence of thioredoxin. Diabetes Res Clin Prac. 1999;45:101–12.
Chang JW, Jeon HB, Lee JH, et al. Augmented expression of peroxiredoxin I in lung cancer. Biochem Biophysic Res Commun. 2001;289:507–12.
Chang TS, Jeong W, Choi SY, Yu S, Kang SW, Rhee SG. Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J Biol Chem. 2002;277:25370–6.
Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–9.
Duyndam MC, Hulscher TM, Fontijn D, Pinedo HM, Boven E. Induction of vascular endothelial growth factor expression and hypoxia-inducible factor 1alpha protein by the oxidative stressor arsenite. J Biol Chem. 2001;276:48066–76.
Finkel T. Oxygen radicals and signaling. Curr Opin Cell Biol. 1998;10:248–53.
Fujii S, Nanbu Y, Nonogaki H, et al. Coexpression of adult T-cell leukemia-derived factor, a human thioredoxin homologue, and human papilloma virus DNA in neoplastic cervical squamous epithelium. Cancer. 1991;68:1583–91.
Gallegos A, Gasdaska JR, Taylor CW, et al. Transfection with human thioredoxin increases cell proliferation and a dominant-negative mutant thioredoxin reverses the transformed phenotype of human breast cancer cells. Cancer Res. 1996;56:5765–70.
Gasdaska JR, Berggren M, Powis G. Cell growth stimulation by the redox protein thioredoxin occurs by a novel helper mechanism. Cell Growth Differ. 1995;6:1643–50.
Gasdaska PY, Oblong JE, Cotgreave IA, Powis G. The predicted amino acid sequence of human thioredoxin is identical to that of the autocrine growth factor human adult T-cell derived factor (ADF): thioredoxin mRNA is elevated in some human tumors. Biochim Biophys Acta 1994;1218:292–6.
Gatenby R, Kessler H, Rosenblum J, et al. Oxygen distribution in squamous cell carcinoma metastases and its relationship to outcome of radiation therapy. Int J Radiat Oncol Biol Phys. 1988;14:831–8.
Graeber TG, Osmanian C, Jacks T, et al. Hypoxia mediated selection of cells with diminished apoptotic potential in solid tumors. Nature(London). 1996;379:88–91.
Güner G, Islekel H, Oto Ö, Hazan, E, Açikel, Ü. Evaluation of some antioxidant enzymes in lung carcinoma tissue. Cancer Lett. 1996;103:233–9.
Haridas V, Ni J, Meager A, et al. TRANK, a novel cytokine that activates NF-κB and c-Jun N-terminal kinase. J Immunol. 1998;161:1–6.
Hirota K, Murata M, Itoh T, Yodoi J, Fukuda K. An endogenous redox molecule, thioredoxin, regulates transactivation of epidermal growth factor receptor and activation of NF-kappaB by lysophosphatidic acid. FEBS Lett. 2001;489:134–8.
Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–15.
Ichimiya S, Davis JG, O'Rourke DM, Katsumata M, Greene MI. Murine thioredoxin peroxidase delays neuronal apoptosis and is expressed in areas of the brain most susceptible to hypoxic and ischemic injury. DNA Cell Biol. 1997;16:311–21.
Ishii T, Yamada M, Sato H, et al. Cloning and characterization of a 23-kDa stress-induced mouse peritoneal macrophage protein. J Biol Chem. 1993;268:18633–6.
Jin DY, Chae HZ, Rhee SG, Jeang KT. Regulatory role for a novel human thioredoxin peroxidase in NF-κB activation. J Biol Chem. 1997;272:30952–61.
Kallman RF. The phenomenon of reoxygenation and its implications for fractionated radiotherapy. Radiology. 1972;105:135–42.
Kang SW, Chae HZ, Seo MS, Kim K, Baines IC, Rhee SG. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-α. J Biol Chem. 1998;273:6297–302.
Karimpour S, Lou J, Lin LL, et al., Thioredoxin reductase regulates AP-1 activity as well as thioredoxin nuclear localization via active cysteines in response to ionizing radiation. Oncogene. 2002;21:6317–27.
Kim TS, Sundaresh CS, Feinstein SI, et al. Identification of a human cDNA clone for lysosomal type Ca2+-independent phospholipase A2 and properties of the expressed protein. J Biol Chem. 1997;272:2542–50.
Kimura H, Braun RD, Ong ET, et al. Fluctuations in red cell flux in tumor microvessels can lead to transient hypoxia and reoxygenation in tumor parenchyma. Cancer Res. 1996;56:5522–8.
Kinnula VL, Lehtonen S, Sormunen R, et al. Overexpression of peroxiredoxins I, II, III, V, and VI in malignant mesothelioma. J Pathol. 2002;196:316–23.
Knowles HJ, Harris AL. Hypoxia and oxidative stress in breast cancer. Hypoxia and tumourigenesis. Breast Cancer Res. 2001;3:318–22.
Koong AC, Denko NC, Hudson KM, et al. Candidate genes for the hypoxic tumor phynotype. Cancer Res. 2000;60:883–7.
Lee KS, Park JS, Kim YJ, et al. Differential expression of Prx I and II in mouse testis and their up-regulation by radiation. Biochem Biophysic Res Commun. 2002;296:337–42.
Liu SL, Lin X, Shi DY, Cheng J, Wu CQ, Zhang YD. Reactive oxygen species stimulated human hepatoma cell proliferation via cross-talk between PI3-K/PKB and JNK signaling pathways. Arch Biochem Biophys. 2002;406:173–82.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–75.
Mahadev K, Wu X, Zilbering A, Zhu L, Lawrence JT, Goldstein BJ. Hydrogen peroxide generated during cellular insulin stimulation is integral to activation of the distal insulin signaling cascade in 3T3-L1 adipocytes. J Biol Chem. 2001;276:48662–9.
Moore RB, Shriver SK. Protein 7.2b of human erythrocyte membranes binds to calpromotin. Biochem Biophysic Res Comm. 1997;232:294–7.
Moore RB, Mankad M, Shriver SK, Mankad VN, Plishker GA. Reconstitution of Ca2+-dependent K+ transport in erythrocyte membrane vesicles requires a cytoplasmic protein. J Biol Chem. 1991;266:18964–8.
Moulder JE, Rockwell S. Tumor hypoxia: its impact on cancer therapy. Cancer Metastasis Rev. 1987;5:313–41.
Noh DY, Ahn SJ, Lee RA, Kim SW, Park IA, Chae HZ. Overexpression of peroxiredoxin in human breast cancer. Anticancer Res. 2001;21:2085–90.
Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41:31–40.
Plishker GA, Chevalier D, Seinsoth L, Moore RB. Calcium-activated potassium transport and high molecular weight forms of calpromotin. J Biol Chem. 1992;267:21839–43.
Prospéri MT, Ferbus D, Karczinski I, Goubin G. A human cDNA corresponding to a gene overexpressed during cell proliferation encodes a product sharing homology with amoebic and bacterial proteins. J Biol Chem. 1993;268:11050–6.
Prospéri MT, Ferbus D, Rouillard D, Goubin G. The pag gene product, a physiological inhibitor of c-ab1 tyrosine kinase, is overexpressed in cells entering S phase and by contact with agents inducing oxidative stress. FEBS Lett. 1998;423:39–44.
Pugh CW, Gleadle J, Maxwell PH. Hypoxia and oxidative stress in breast cancer. Hypoxia signalling pathway. Breast Cancer Res. 2001;3:313–7.
Rabilloud T, Berthier R, Vincon M, Ferbus D, Goubin G, Lawrence JJ. Early events in erythroid differentiation: accumulation of the acidic peroxiredoxin (PRP/TSA/NKEF-B). Biochem J. 1995;312:699–705.
Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6.
Rofstad EK, Danielson T. Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor mediated angiogenesis. Br J Cancer 1999;80:1697–707.
Sauri H, Ashjian PH, Kim AT, Shau H. Recombinant natural killer enhancing factor augments natural killer cytotoxicity. J Leukoc Biol. 1996;59:925–31.
Schwickert G, Walenta S, Sundfor K, Rofstad EK, Mueller-Klieser W. Correlation of high lactate levels in human cervical cancer with incidence of metastasis. Cancer Res. 1995;55:4757–9.
Shen C, Nathan C. Nonredundant antioxidant defense by multiple two-cysteine peroxiredoxins in human prostate cancer cells. Mol Med. 2002;8:95–102.
Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–9.
Teicher B, Lazo J, Sartorelli A. Classification of antineoplastic agents by their selective toxicities toward oxygenated and hypoxic tumor cells. Cancer Res. 1981;41:73–81.
Teicher B, Lazo J, Sartorelli A. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–68.
Vaupel P, Kallinowski F, Okunief, P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–65.
Voet D, Voet JG. Biochemistry. New York: John Wiley & Sons. 1990:337.
Williams KJ, Cowen RL, Stratford IJ. Hypoxia and oxidative stress. Tumour hypoxia -- therapeutic considerations. Breast Cancer Res. 2001;3:328–31.
Young SD, Hill RF. Effects of reoxigenation on cells from hypoxic regions of solid tumors: anticancer drug sensitivity and metastatic potential. J Natl Cancer Inst. 1990;82:371–80.
Zhang P, Liu B, Kang SW, Seo MS, Rhee SG, Obeid LM. Thioredoxin peroxidase is a novel inhibitor of apoptosis with a mechanism distinct from that of Bcl-2. J Biol Chem. 1997;272:30615–18.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kim, H., Chae, HZ., Kim, YJ. et al. Preferential elevation of Prx I and Trx expression in lung cancer cells following hypoxia and in human lung cancer tissues. Cell Biol Toxicol 19, 285–298 (2003). https://doi.org/10.1023/B:CBTO.0000004952.07979.3d
Issue Date:
DOI: https://doi.org/10.1023/B:CBTO.0000004952.07979.3d