Abstract
The heterogeneous asymmetric ring opening (ARO) ofmeso-epoxides with trimethylsilylazide (TMSN3) catalysed by a Cr(salen) catalyst coordinated on an aminopropyl functionalised silica material combines good enantioselectivities with high conversions and suffers low leaching (<1%).
Similar content being viewed by others
References
D. M. Hodgson, A. R. Gibbs and G. P. Lee,Tetrahedron 52 (1996) 14361.
(a)J. Wu, X. -L. Hou, L. -X. Dai, L. -J. Xia and M. -H. Tang, Tetrahedron Asymm. 9 (1998) 3431;(b)X. -L. Hou,J. Wu,L. -X. Dai,L. -J. Xia and M. -H. Tang,Tetrahedron Asymm. 9 (1998) 1747;(c)N. Oguni,Y. Miyagi and K. Itoh,Tetrahedron Lett. 39 (1998)9023;(d)M. Nakajima,M. Saito,M. Uemura and S. Hashimoto,Tetrahedron Lett. 43 (2002)8827;(e)W. A. Nugent,J. Am. Chem. Soc. 114 (1992)2768.
(a)L. E. Martinez, J. L. Leighton, D. H. Carsten and E. N. Jacobsen,J. Am. Chem. Soc. 117 (1995) 5897;(b)S. E. Schaus, J. F. Larrow and E. N. Jacobsen,J. Org. Chem. 62 (1997)4197; (c)E. N. Jacobsen,Acc. Chem. Res. 33 (2000)421.
C. Baleizaö, B. Gigante, M. J. Sabater, H. Garcia and A. Corma, Appl. Catal. A:Gen. 228 (2002)279.
M. D. Angelino and P. E. Laibinis,J. Polym. Sci. Part A. Polym. Chem. 37 (1999)3888.
B. Gigante, A. Corma, H. Garcia and M. J. Sabater,Catal. Lett. 68 (2000)113.
B. M. L. Dioos and P. A. Jacobs,Tetrahedron Lett. 44 (2003) 8815. [8]_The procedure used for the preparation of the catalyst is based on references [4]and [12]with some small modi cations. [9]_(R,R)-Cr III (salen)catalyst was bought from Aldrich. [10]_Silica gel 60 for preparative column chromatography was bought from Fluka. [11]_All enantiomeric/diastereomeric excesses were determined by chiral GC on a Chrompack-CHIRASIL-DEX CB column (0. 32 mm 0. 25 l 25 m)using FID detection.
The procedure used for the preparation of the catalyst is based on references [4 ]and [12 ]with some small modi cations.
(R,R)-Cr III (salen)catalyst was bought from Aldrich.
Silica gel 60 for preparative column chromatography was bought from Fluka.
All enantiomeric/diastereomeric excesses were determined by chiral GC on a Chrompack-CHIRASIL-DEX CB column (0.32 mm 0.25 l 25 m)using FID detection.
X. -G. Zhou, X. -Q. Yu, J. -S. Huang, S. -G. Li, L. -S. Li and C. -M. Che,Chem. Commun. 18 (1999)1789.
The Cr(salen)complex is insoluble in noncoordinating solvents but readily soluble in donor solvents such as ethereal solvents K. B. Hansen, J. L. Leighton and E. N. Jacobsen, J. Am. Chem. Soc. 118 (1996) 10924.
B. M. L. Dioos and P. A. Jacobs,Tetrahedron Lett. 44 (2003) 4715.
Cr3+ ions have two absorption bands at about 430 and 614 nm due to d-d transitions.
In a recycling experiment for the ARO of epoxyhexane over three consecutive runs,leaching increased from 2% in the rst run to 5 and 8%in the second and third run respectively. An analogous increase in leaching is found for epoxyoctane.
Depending on ligand type,hexacoordinate Cr3+ species are relatively inert.
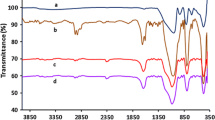
The IR analyses were performed on KBr samples of the silica material used for 10 runs.
The imine stretch signal is found at 1620 cm-1 as well,but its intensity is small in comparison with the NH2 bending signal. Therefore the resulting IR absorption in this region is mainly due to the NH2 bending.
The IR absorption signal at 2100 cm-1 corresponds with an organic azide stretch. This can be due to ring opened product still bound to the catalyst or ring opened product adsorbed on the silica material. Nevertheless,because of the small excess of TMSN3 present in the reaction mixture,a small shoulder can be observed at 2050 cm-1,typically for the active Cr(salen)N3 species [13].
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dioos, B.M., Geurts, W.A. & Jacobs, P.A. Coordination of CrIII(Salen) on Functionalised Silica for Asymmetric Ring Opening Reactions of Epoxides. Catalysis Letters 97, 125–129 (2004). https://doi.org/10.1023/B:CATL.0000038573.81490.03
Issue Date:
DOI: https://doi.org/10.1023/B:CATL.0000038573.81490.03